Last update images today Adverse Event Reporting In Clinical Trials



















+report+both+incidents+and+near+misses+to+SHOT..jpg)

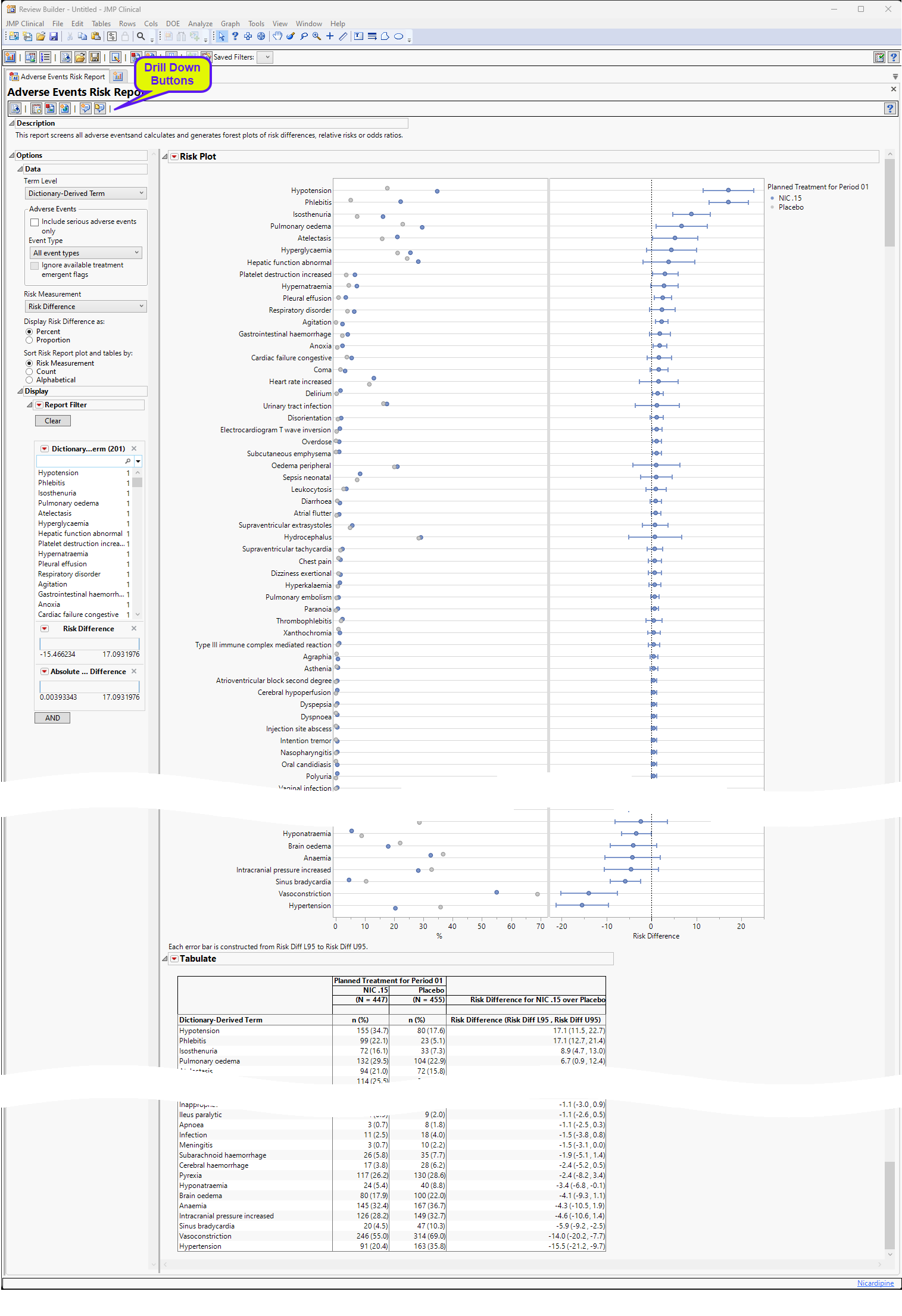











https www jmp com support downloads JMPC172 documentation Content Resources Images AdverseEventsRiskReport 1 png - Adverse Events Risk Report AdverseEventsRiskReport 1 https www researchgate net profile Sahil Garg 12 publication 357196053 figure tbl2 AS 1179548024619012 1658237572802 Adverse events in the trial population during hospital stay png - Adverse Events In The Trial Population During Hospital Stay Download Adverse Events In The Trial Population During Hospital Stay
https www researchgate net publication 284126612 figure tbl2 AS 667815501639683 1536231031195 Adverse Events Reported in Each Clinical Trial png - Adverse Events Reported In Each Clinical Trial Download Table Adverse Events Reported In Each Clinical Trial https covers tandf co uk og USBR png - Comparison Of Adverse Event Risks In Randomized Controlled Trials With USBR https www bplogix com hs fs hubfs Adverse Event Reporting Challenges png - Adverse Event Reporting Challenges How To Overcome Them Adverse Event Reporting Challenges
https s2 studylib net store data 005594811 1 621b3e6f370dcef905b8d62255db2d1c 768x994 png - Managing Clinical Adverse Events 005594811 1 621b3e6f370dcef905b8d62255db2d1c 768x994 https www researchgate net publication 368475643 figure tbl1 AS 11431281120354622 1676472089695 Summary of Treatment Emergent Adverse Events in the Safety Analysis Population png - Summary Of Treatment Emergent Adverse Events In The Safety Analysis Summary Of Treatment Emergent Adverse Events In The Safety Analysis Population
https www viedoc com site assets files 10904 expect 2024 4 1000x0 jpg - What To Expect In The Clinical Trials Industry In 2024 Viedoc Expect 2024 4.1000x0
https slideplayer com slide 15308026 92 images 17 Adverse event reporting jpg - Clinical Pharmacologic Evaluation Of Adverse Events Ppt Download Adverse Event Reporting https www frontiersin org files Articles 874829 fimmu 13 874829 HTML image m fimmu 13 874829 t003 jpg - Frontiers Adverse Event Reporting Quality In Cancer Clinical Trials Fimmu 13 874829 T003
https www researchgate net publication 351951334 figure tbl1 AS 1028608478965761 1622250780293 FDA Adverse Event Reporting System analysis case demographics png - FDA Adverse Event Reporting System Analysis Case Demographics FDA Adverse Event Reporting System Analysis Case Demographics https covers tandf co uk og USBR png - Comparison Of Adverse Event Risks In Randomized Controlled Trials With USBR
https slideplayer com slide 16371523 95 images 10 Adverse Events All respondents 16 report both incidents and near misses to SHOT jpg - North Thames Regional Transfusion Committee Ppt Download Adverse Events All Respondents (16) Report Both Incidents And Near Misses To SHOT. https www researchgate net publication 270002849 figure tbl1 AS 669139567915013 1536546713088 Serious adverse events reported during the trial png - Serious Adverse Events Reported During The Trial Download Table Serious Adverse Events Reported During The Trial https www centerwatch com ext resources products Clinical Trials Adverse Event Reporting Guide 2022 500 jpg - Clinical Trials Adverse Event Reporting Guide 2022 Edition CenterWatch Clinical Trials Adverse Event Reporting Guide 2022 500
https www researchgate net publication 368475643 figure tbl1 AS 11431281120354622 1676472089695 Summary of Treatment Emergent Adverse Events in the Safety Analysis Population png - Summary Of Treatment Emergent Adverse Events In The Safety Analysis Summary Of Treatment Emergent Adverse Events In The Safety Analysis Population https jackson medical com wp content uploads 2020 11 AdobeStock 347720224 1024x683 jpeg - 4 Key Benefits Of Reporting Adverse Events GloShield AdobeStock 347720224 1024x683
https journals sagepub com cms 10 1177 CLAA 56 18 asset 1665cad3 7e16 5cad 57e1 65cad357e166 claa 56 18 largecover png - Reporting On Adverse Clinical Events 2018 Claa 56 18.largecover
https www researchgate net publication 347253149 figure tbl4 AS 969410730790916 1608136937911 Adverse events reported in the included trials png - Adverse Events Reported In The Included Trials Download Scientific Adverse Events Reported In The Included Trials https www frontiersin org files Articles 874829 fimmu 13 874829 HTML image m fimmu 13 874829 t003 jpg - Frontiers Adverse Event Reporting Quality In Cancer Clinical Trials Fimmu 13 874829 T003
https assets website files com 5ebe569becbcbc7848cc9c42 6332c3d6922d271a5cf9ec0c How to ensure AE reporting blog png - How To Ensure Efficient And Compliant Adverse Event Reporting Under The 6332c3d6922d271a5cf9ec0c How To Ensure AE Reporting Blog https www frontiersin org files Articles 874829 fimmu 13 874829 HTML image m fimmu 13 874829 t003 jpg - Frontiers Adverse Event Reporting Quality In Cancer Clinical Trials Fimmu 13 874829 T003
https s2 studylib net store data 005594811 1 621b3e6f370dcef905b8d62255db2d1c 768x994 png - Managing Clinical Adverse Events 005594811 1 621b3e6f370dcef905b8d62255db2d1c 768x994 https www researchgate net publication 368475643 figure tbl1 AS 11431281120354622 1676472089695 Summary of Treatment Emergent Adverse Events in the Safety Analysis Population png - Summary Of Treatment Emergent Adverse Events In The Safety Analysis Summary Of Treatment Emergent Adverse Events In The Safety Analysis Population https www jmp com support downloads JMPC80 documentation Content Resources Images AdverseEventsReport 1b png - Adverse Events Report AdverseEventsReport 1b
https www dpo uab edu medicine exercise images REACT Materials Serious adverse event reporting png - Clinical Trial Resources School Of Medicine Center For Exercise Serious Adverse Event Reporting https www centerwatch com ext resources images editorial AdverseEvents 360x240 png - Stop Over Reporting Adverse Events FDA Officials Tell Sponsors 2021 AdverseEvents 360x240
https slideplayer com slide 16371523 95 images 10 Adverse Events All respondents 16 report both incidents and near misses to SHOT jpg - North Thames Regional Transfusion Committee Ppt Download Adverse Events All Respondents (16) Report Both Incidents And Near Misses To SHOT.