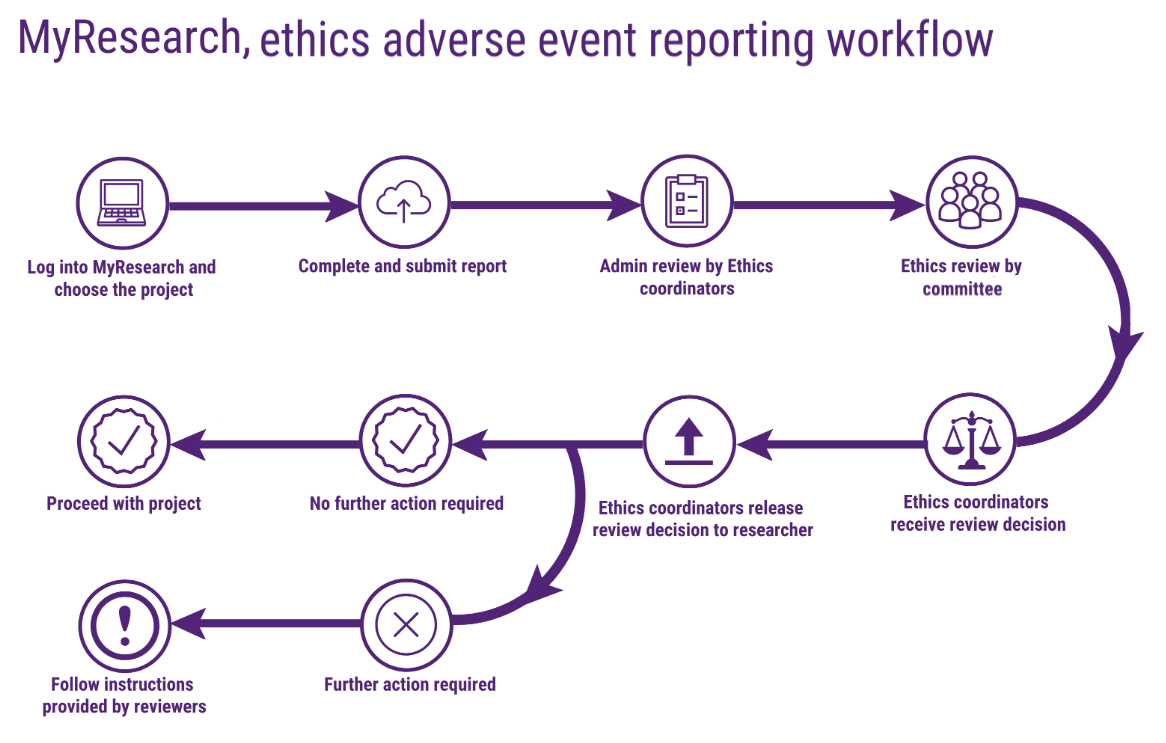
Last update images today Adverse Events Reporting


























https www researchgate net publication 259208375 figure fig5 AS 340417460621318 1458173255413 Reporting of adverse events at ClinicalTrialsgov and in published articles png - Reporting Of Adverse Events At ClinicalTrials Gov And In Published Reporting Of Adverse Events At ClinicalTrialsgov And In Published Articles https s2 studylib net store data 011642223 1 a0dc2a18df06cb5e7e52fc21c257a9d6 png - adverse form reporting events serious sae studylib Serious Adverse Events Reporting Form 011642223 1 A0dc2a18df06cb5e7e52fc21c257a9d6
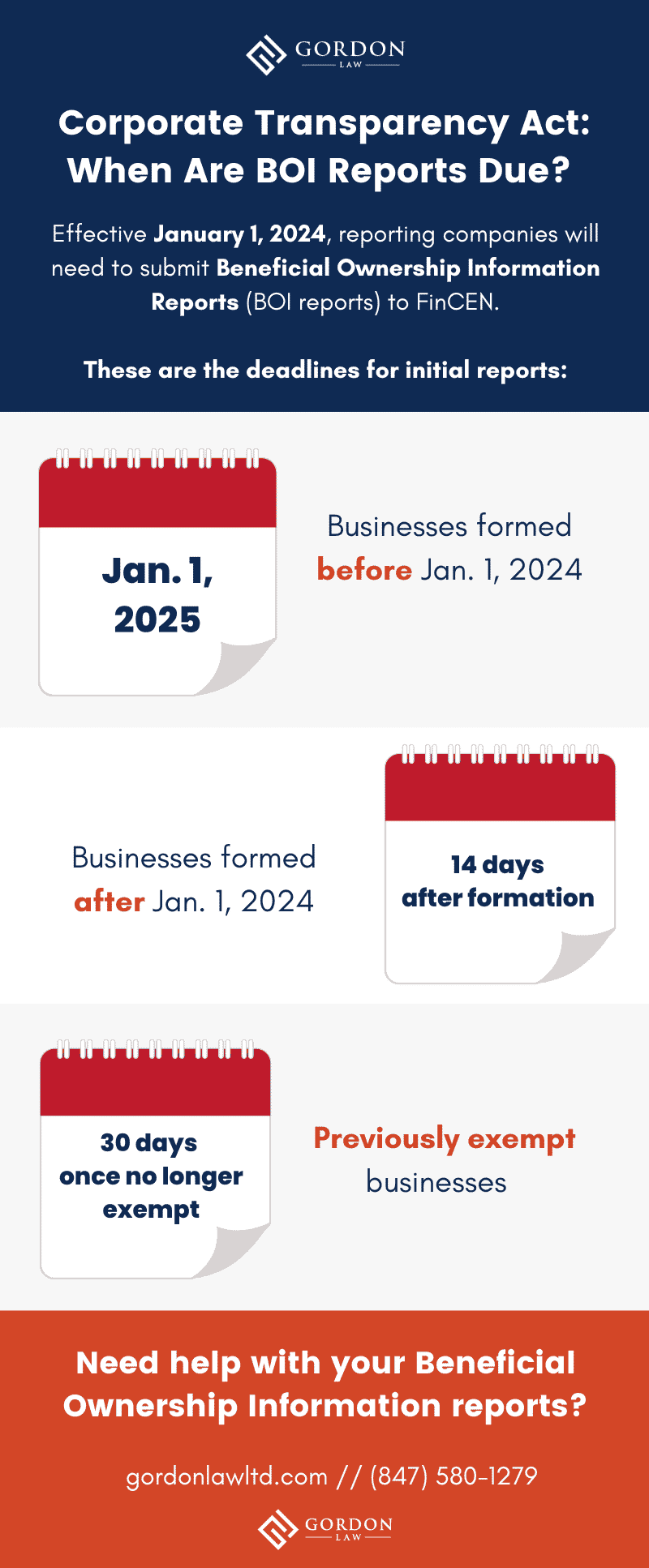
https ai2 s2 public s3 amazonaws com figures 2017 08 08 709624368d051467902264da545e390c7d7af4d3 4 Figure2 1 png - Figure 2 From The Automation Of Clinical Trial Serious Adverse Event 4 Figure2 1 https d2908q01vomqb2 cloudfront net fc074d501302eb2b93e2554793fcaf50b3bf7291 2023 02 13 adverse 1 png - Automating Adverse Events Reporting For Pharma With Amazon Connect And Adverse 1 https gordonlawltd com wp content uploads 2022 04 beneficial ownership information report deadlines png - Who Won Act 2024 Bria Marlyn Beneficial Ownership Information Report Deadlines
https s3 studylib net store data 006842670 1 b1b27eaa6dcba47fccd554f0ccc5efe1 png - form adverse reporting events serious sae clinical trials research Serious Adverse Events Reporting Form For 006842670 1 B1b27eaa6dcba47fccd554f0ccc5efe1 https www cbh com wp content uploads 2023 04 Article Recent RD Tax Credit Cases Highlight Importance 1093056922 Facebook jpg - BOI Reporting Regulations Effective January 2024 Cherry Bekaert Article Recent RD Tax Credit Cases Highlight Importance 1093056922 Facebook
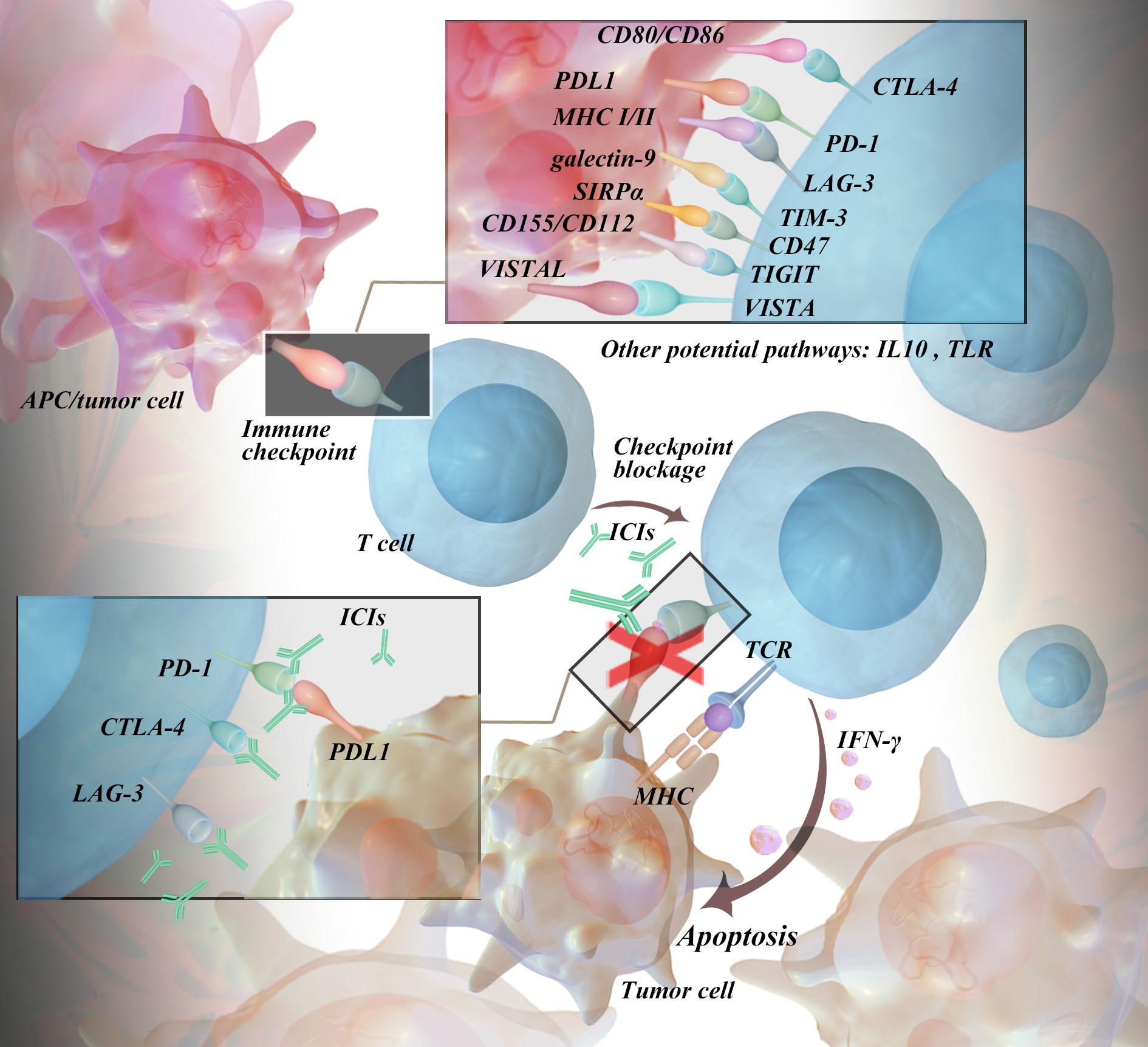
https assets global website files com 621e95f9ac30687a56e4297e 65dfad77d6925f1ae59a0c75 V2 1693538989084 5002d1c3 b337 4d95 99ae 4e83dfea7d87 HIGH RES png - Mechanisms Of Immune Related Adverse Events After Immune Checkpoint 65dfad77d6925f1ae59a0c75 V2 1693538989084 5002d1c3 B337 4d95 99ae 4e83dfea7d87 HIGH RES
https uha blob core windows net accelerate attachments cjvpqgbxz06af0gpc3cviznrl safety events 01 full png - Health And Safety Events Calendar 2024 Cynde Rodina Cjvpqgbxz06af0gpc3cviznrl Safety Events 01.full https journals sagepub com cms 10 1177 CLAA 62 1 2 asset 18cde74e 8118 de74 4811 cde74e48118c claa 62 1 2 largecover png - Reporting On Adverse Clinical Events 2024 Claa 62 1 2.largecover
https s2 studylib net store data 011642223 1 a0dc2a18df06cb5e7e52fc21c257a9d6 png - adverse form reporting events serious sae studylib Serious Adverse Events Reporting Form 011642223 1 A0dc2a18df06cb5e7e52fc21c257a9d6 https journals sagepub com cms 10 1177 CLAA 62 1 2 asset 18cde74e 8118 de74 4811 cde74e48118c claa 62 1 2 largecover png - Reporting On Adverse Clinical Events 2024 Claa 62 1 2.largecover
https www cisema com wp content uploads 2020 12 Adverse Events jpg - adverse reporting guidelines devices Guidelines For Reporting Adverse Events For Medical Devices Adverse Events https d2908q01vomqb2 cloudfront net fc074d501302eb2b93e2554793fcaf50b3bf7291 2023 02 13 adverse 1 png - Automating Adverse Events Reporting For Pharma With Amazon Connect And Adverse 1 https med virginia edu policies wp content uploads sites 415 2020 12 GeneTransferSAEReportingForm2 2 1 1161x1536 jpg - Serious Adverse Event Reporting Form Gene Transfer Protocol Policies GeneTransferSAEReportingForm2 2 1 1161x1536
https www researchgate net publication 259208375 figure fig5 AS 340417460621318 1458173255413 Reporting of adverse events at ClinicalTrialsgov and in published articles png - Reporting Of Adverse Events At ClinicalTrials Gov And In Published Reporting Of Adverse Events At ClinicalTrialsgov And In Published Articles https ai2 s2 public s3 amazonaws com figures 2017 08 08 709624368d051467902264da545e390c7d7af4d3 4 Figure2 1 png - Figure 2 From The Automation Of Clinical Trial Serious Adverse Event 4 Figure2 1
https www duurzaam ondernemen nl wordpress wp content uploads 2024 01 TFOCR2024 Intire banner png - The Future Of Corporate Reporting 2024 Duurzaam Ondernemen TFOCR2024.Intire.banner
https www cbh com wp content uploads 2023 04 Article Recent RD Tax Credit Cases Highlight Importance 1093056922 Facebook jpg - BOI Reporting Regulations Effective January 2024 Cherry Bekaert Article Recent RD Tax Credit Cases Highlight Importance 1093056922 Facebook https hobartoccupationalmedicine com au wp content uploads 2016 03 images 10 jpeg - adverse reporting event events introduced affecting systematically workers practice record being march system has Information About Adverse Event Reporting Images 10
https www accestra com wp content uploads 2022 12 Collection Reporting of Individual jpg - China Pharmacovigilance Collection Reporting Of Individual Adverse Collection Reporting Of Individual https d2908q01vomqb2 cloudfront net fc074d501302eb2b93e2554793fcaf50b3bf7291 2023 02 13 adverse 1 png - Automating Adverse Events Reporting For Pharma With Amazon Connect And Adverse 1
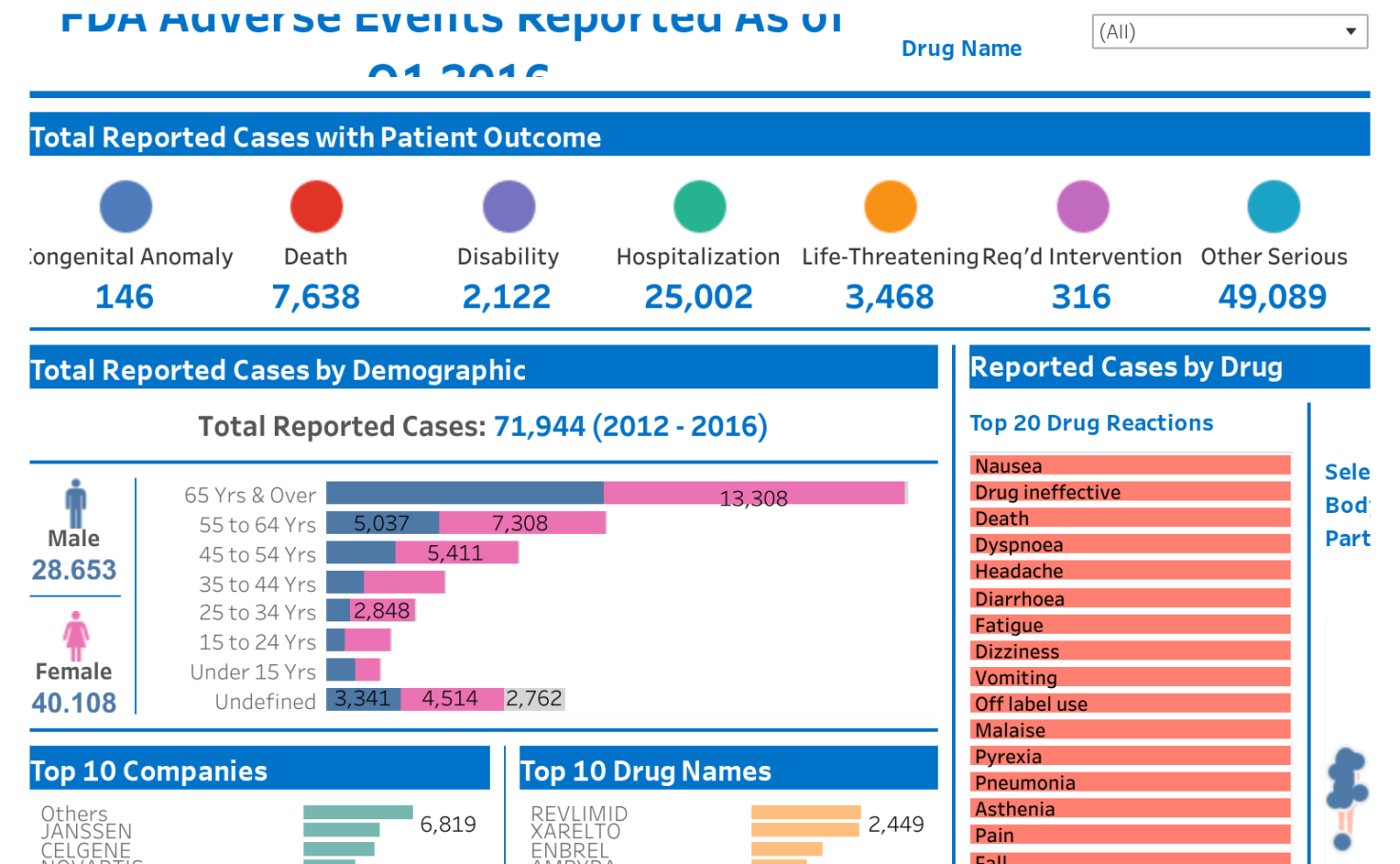
https med virginia edu policies wp content uploads sites 415 2020 12 GeneTransferSAEReportingForm2 2 1 1161x1536 jpg - Serious Adverse Event Reporting Form Gene Transfer Protocol Policies GeneTransferSAEReportingForm2 2 1 1161x1536 https bundle report wp content uploads sites 22 2024 01 2024 Dates Deadlines Calendar o5iZ3A png - 2024 Quality Reporting Deadlines Calendar Bundle Report 2024 Dates Deadlines Calendar O5iZ3A https public tableau com static images FD FDAAdverseEvent Final FDAAdverseEventsDashboard 4 3 hd png - adverse dashboard FDA Adverse Events Dashboard Tableau Public 4 3 Hd
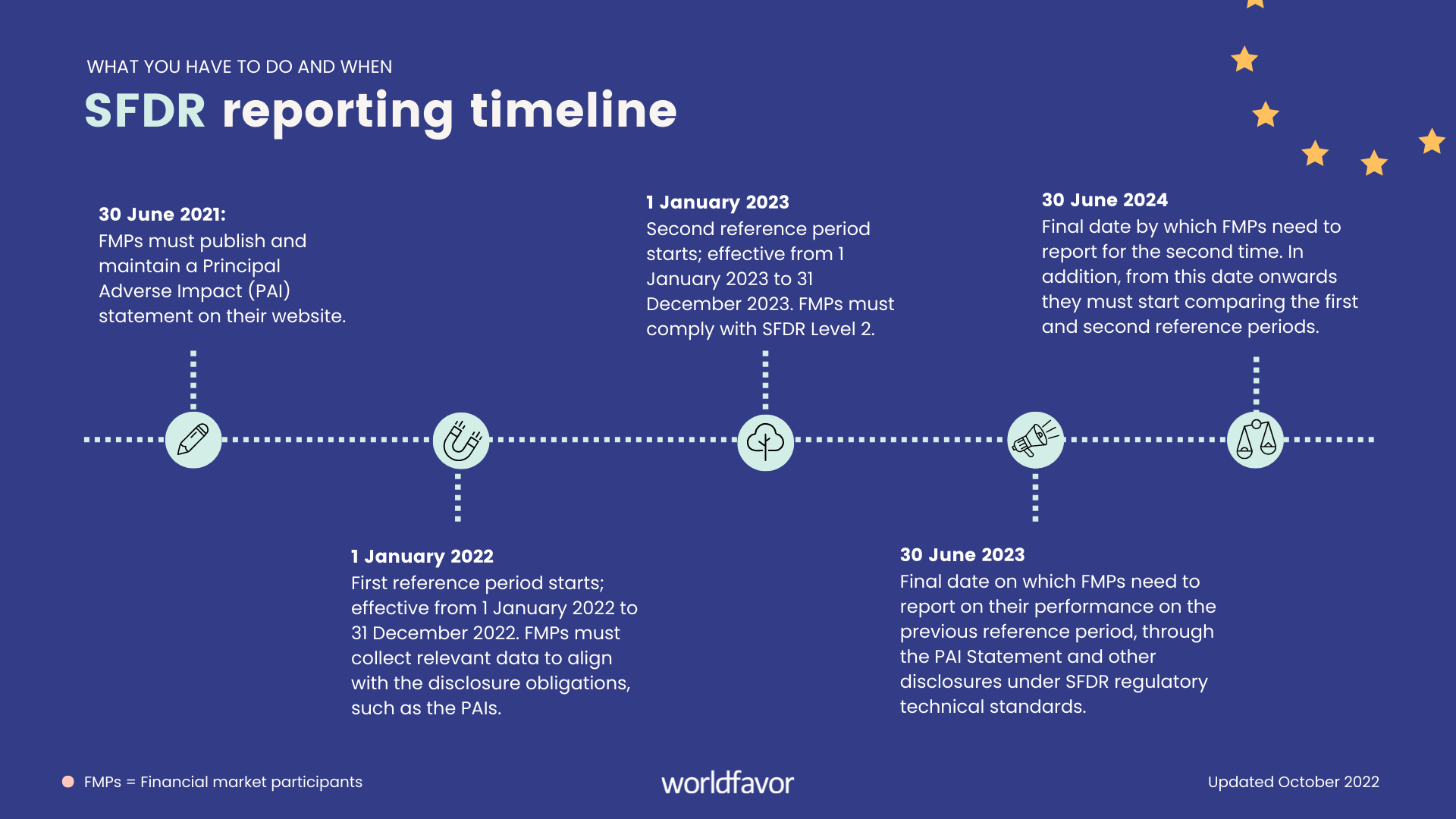
https blog worldfavor com hs fs hubfs Blog pictures SFDR reporting timeline what you have to disclose and when png - SFDR Reporting Timeline What You Have To Disclose And When SFDR Reporting Timeline What You Have To Disclose And When https www cbh com wp content uploads 2023 04 Article Recent RD Tax Credit Cases Highlight Importance 1093056922 Facebook jpg - BOI Reporting Regulations Effective January 2024 Cherry Bekaert Article Recent RD Tax Credit Cases Highlight Importance 1093056922 Facebook
https www bplogix com hs fs hubfs Adverse Event Reporting Challenges png - Adverse Event Reporting Ensuring Patient Safety In Medical Device And Adverse Event Reporting Challenges