Last update images today Eu Mdr Annex Ii























https medtechintelligence com wp content uploads 2018 01 EUMDR ComplianceRequirements jpg - 1 What Is The EU MDR In Depth Explanation Of The Regulation 58 OFF EUMDR ComplianceRequirements http www orielstat com blog wp content uploads 2021 03 eumdr graphic e1614962121251 jpg - 1 Class 1 Medical Device Requirements Oriel STAT A MATRIX Eumdr Graphic E1614962121251
https www rcainc com wp content uploads 2018 09 RCA EU MDR Overview Compliance and Implementation Strategy Services Page 1 jpg - 1 EU MDR Compliance And EU MDR Implementation Strategy Services RCA EU MDR Overview Compliance And Implementation Strategy Services Page 1 https operonstrategist com wp content uploads 2022 11 Traceability requirements for Medical Devices in EU MDR 01 1024x535 jpg - 1 Traceability Requirements For Medical Devices In EU MDR Operon Strategist Traceability Requirements For Medical Devices In EU MDR 01 1024x535 https i ytimg com vi SXQcYHWfLxY maxresdefault jpg - 1 Short EU MDR Commission Extension Proposal Medicaldevice Maxresdefault
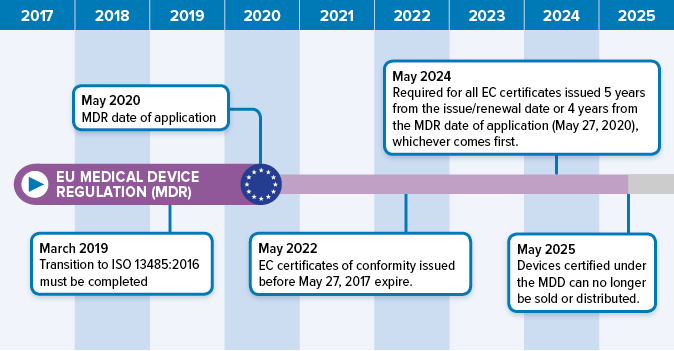
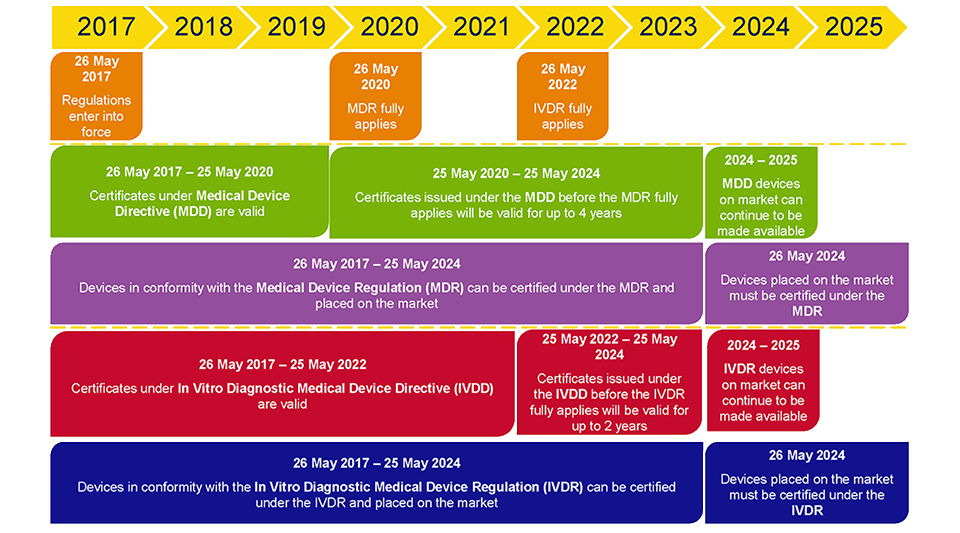
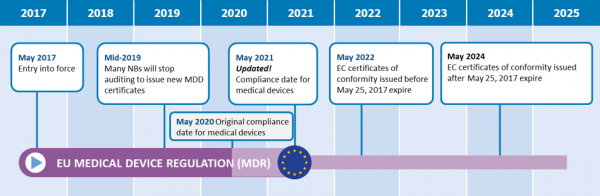
https cemarking net wp content uploads 2014 06 Bigstock 24079583 European Commission jpg - 1 Medical Device Regulation MDR New Guidance Documents Published Bigstock 24079583 European Commission https www orielstat com blog wp content uploads 2018 07 EU MDR Timeline 2 png - 1 Preparing For The EU MDR 2020 Changes Oriel STAT A MATRIX EU MDR Timeline 2
https cemarking net wp content uploads 2022 04 Changes new MDR jpg - 1 EU MDR 2017 745 What Changed Changes New MDR
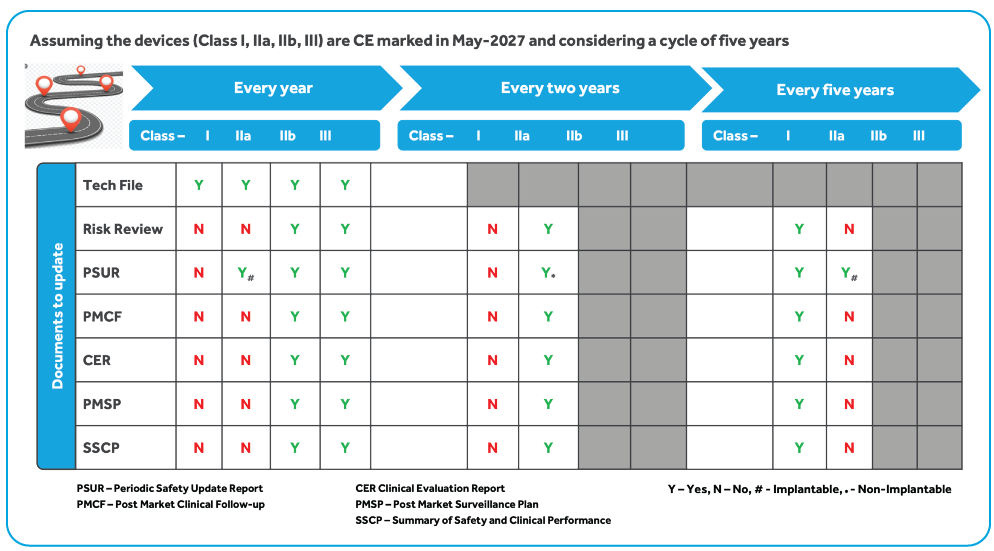
https www celegence com wp content uploads 2021 06 Post Market Surveillance EU MDR Celegence Feature 500x383 2x jpg - 1 EU MDR Checklist Timelines Impact On Medical Device Companies Post Market Surveillance EU MDR Celegence Feature 500x383@2x https med di dia com includes items images intro 2849 400x250 eu mdr annex 3 png - 1 Annex III EU MDR 2849 400x250 Eu Mdr Annex 3
https gemarmed com wp content uploads 2021 11 CE EU Medical Device Regulation MDR Gemarmed jpg - 1 Getting CE Marking With EU MDR Requirements GEMARMED CE EU Medical Device Regulation MDR Gemarmed https www cyient com hubfs Whitepaper medical device regulatory affairs EU MDR Sustenance Roadmap png - 1 Whitepaper Overview Of Medical Device Regulatory Affairs EU MDR Sustenance Roadmap
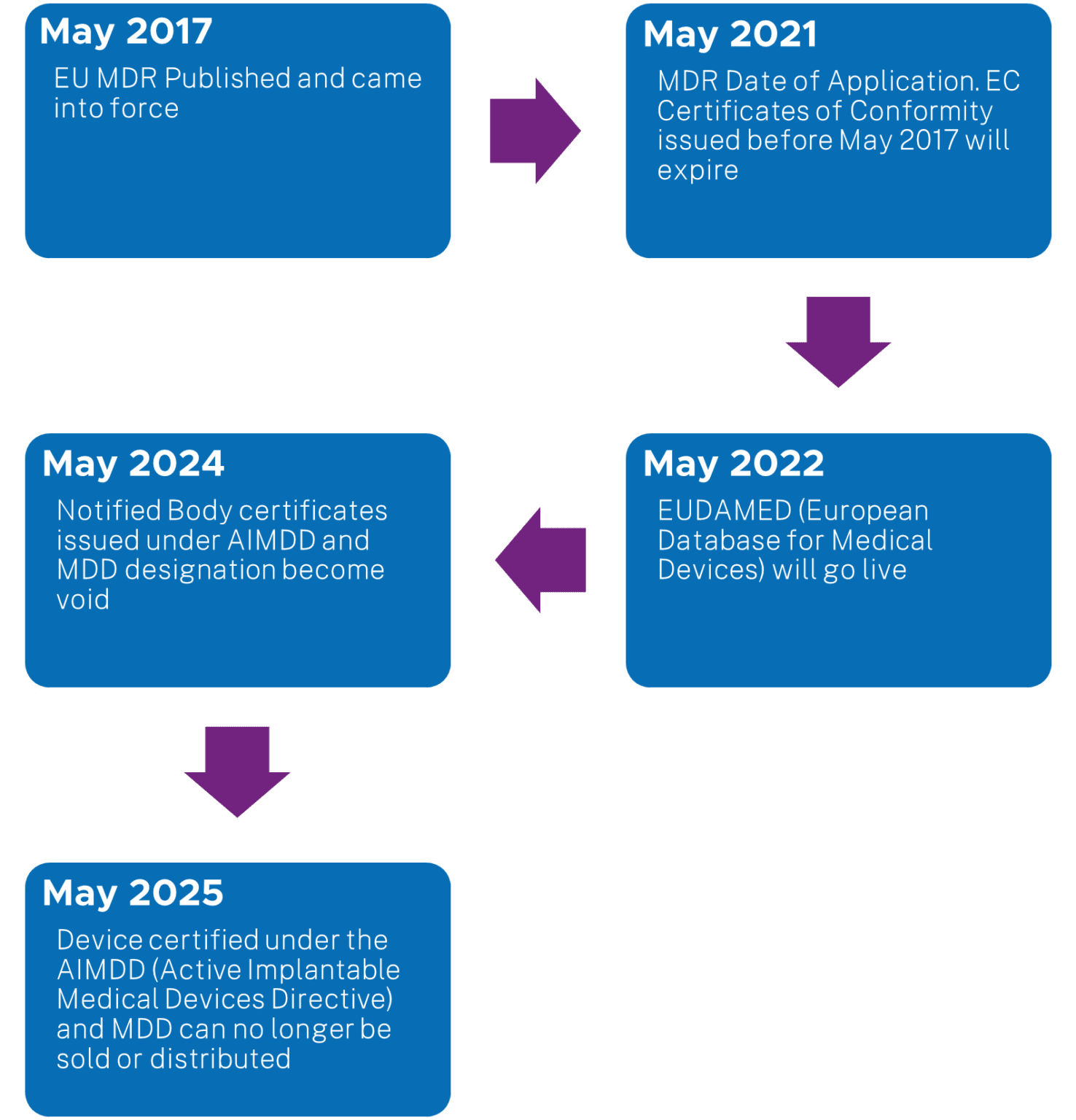
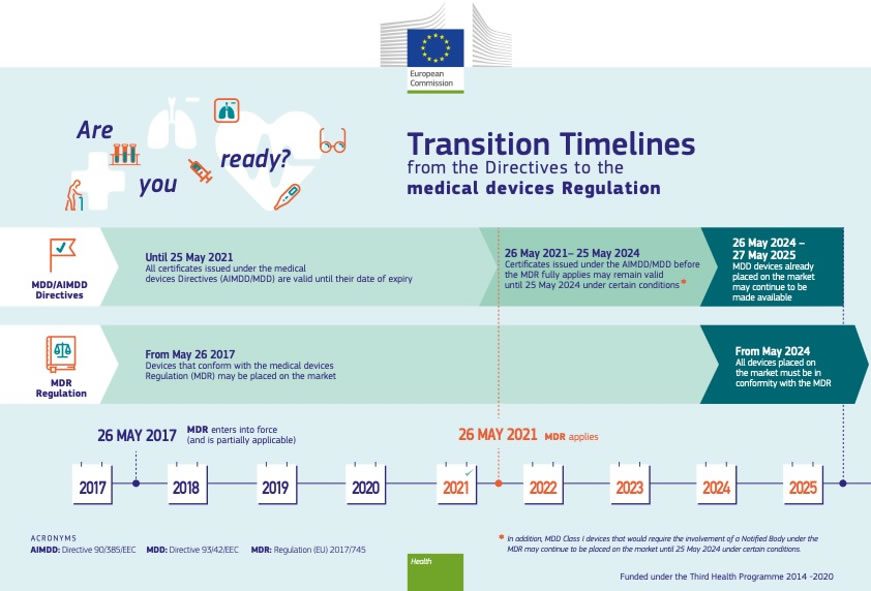
https www orielstat com blog wp content uploads 2018 07 EU MDR Timeline 2 png - 1 Preparing For The EU MDR 2020 Changes Oriel STAT A MATRIX EU MDR Timeline 2 https www mylanguageconnection com wp content uploads 2020 01 EU MDR Timeline png - 1 The EU Medical Device Regulation EU MDR My Language Connection EU MDR Timeline https www regulatoryglobe com wp content uploads 2020 11 Transition article 120 jpg - 1 Medical Device Regulation EU MDR Implementation Guide Transition Article 120
https www orielstat com blog wp content uploads 2019 02 EUMDR NewTimeline2 e1591716276456 png - 1 EU MDR Transition Timelines And Deadlines For 2017 745 EUMDR NewTimeline2 E1591716276456 https www argosmultilingual com wp content uploads 2020 04 EU MDR Strategy and Checklist Whitepaper jpg - 1 EU MDR Post Deadline Announcement Strategy Argos Multilingual EU MDR Strategy And Checklist Whitepaper
https emmainternational com wp content uploads 2020 02 EUMDRSimplified Blog png - 1 Understanding Annex I Of EU MDR EUMDRSimplified Blog
https www qualitymeddev com wp content uploads 2021 09 Screenshot 2021 09 19 at 19 00 39 800x1128 png - 1 Harmonised Standards And EU MDR 2017 745 Screenshot 2021 09 19 At 19.00.39 800x1128 https www celegence com wp content uploads 2021 06 Post Market Surveillance EU MDR Celegence Feature 500x383 2x jpg - 1 EU MDR Checklist Timelines Impact On Medical Device Companies Post Market Surveillance EU MDR Celegence Feature 500x383@2x
https www elexes com wp content uploads 2023 07 EU MDR transition period finalized png - 1 Extension To EU MDR Transition Period Finalized Elexes EU MDR Transition Period Finalized https i ytimg com vi SXQcYHWfLxY maxresdefault jpg - 1 Short EU MDR Commission Extension Proposal Medicaldevice Maxresdefault
https www regulatoryglobe com wp content uploads 2018 11 MDR Overview 1 neu 600x391 png - 1 EU MDR Implementation Guide For Medical Devices MDCG MDR Overview 1 Neu 600x391 https precision lifesciences com wp content uploads 2022 11 EU blog header large jpg - 1 EU MDR Transition Tasks Precision Life Sciences EU Blog Header Large https medtechintelligence com wp content uploads 2018 01 EUMDR ComplianceRequirements jpg - 1 What Is The EU MDR In Depth Explanation Of The Regulation 58 OFF EUMDR ComplianceRequirements
https www greenlight guru hs fs hubfs header EU MDR implementation guide1 png - 1 EU MDR Implementation Guide Header EU MDR Implementation Guide1 http www greensofttech com wp content uploads 2018 08 EU MDR featured image png - 1 Compliance With New EU MDR In 2020 GreenSoft Technology EU MDR Featured Image
https www celegence com wp content uploads 2021 06 Post Market Surveillance EU MDR Celegence Feature 500x383 2x jpg - 1 EU MDR Checklist Timelines Impact On Medical Device Companies Post Market Surveillance EU MDR Celegence Feature 500x383@2x