Last update images today Eu Mdr Common Specifications

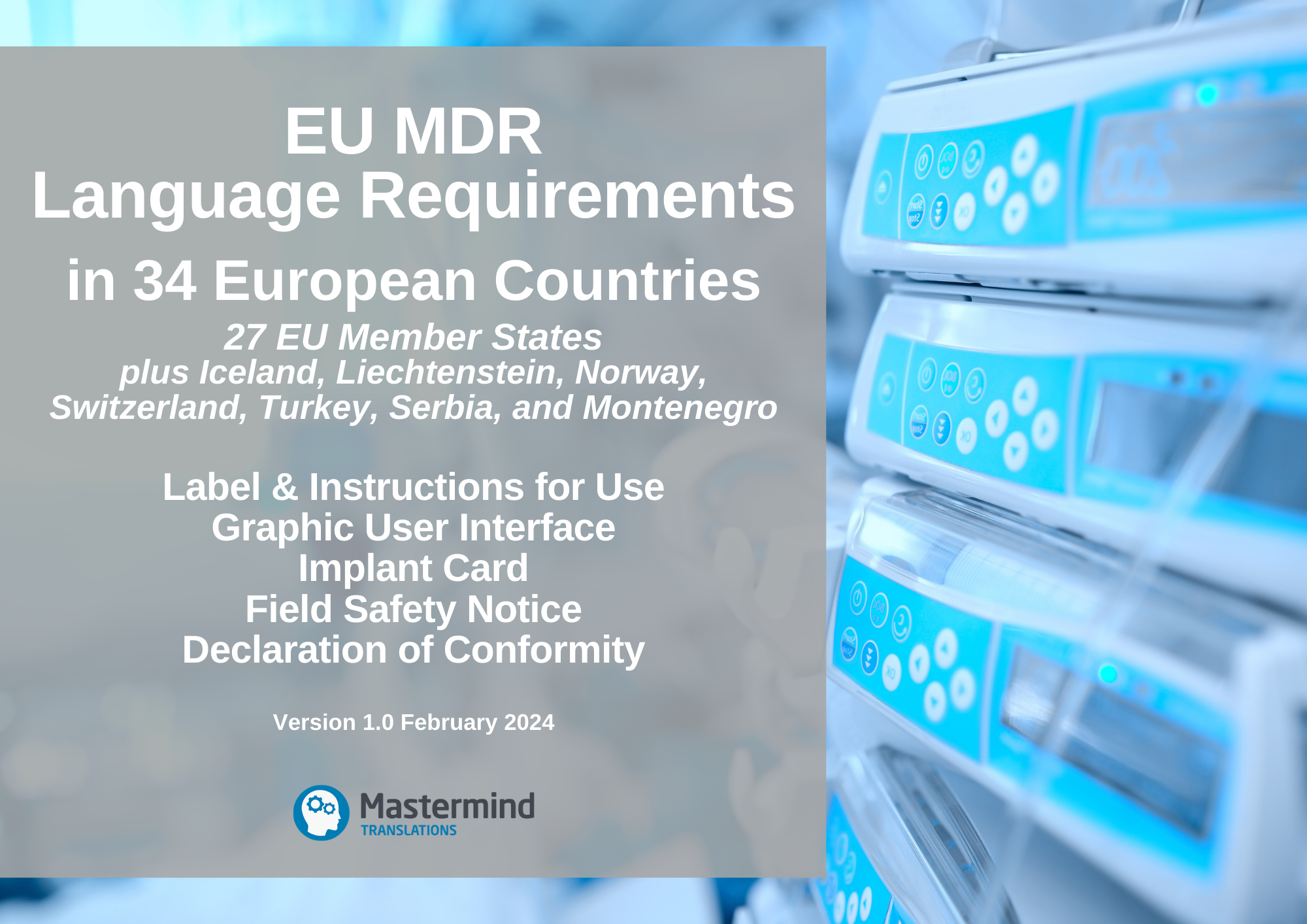











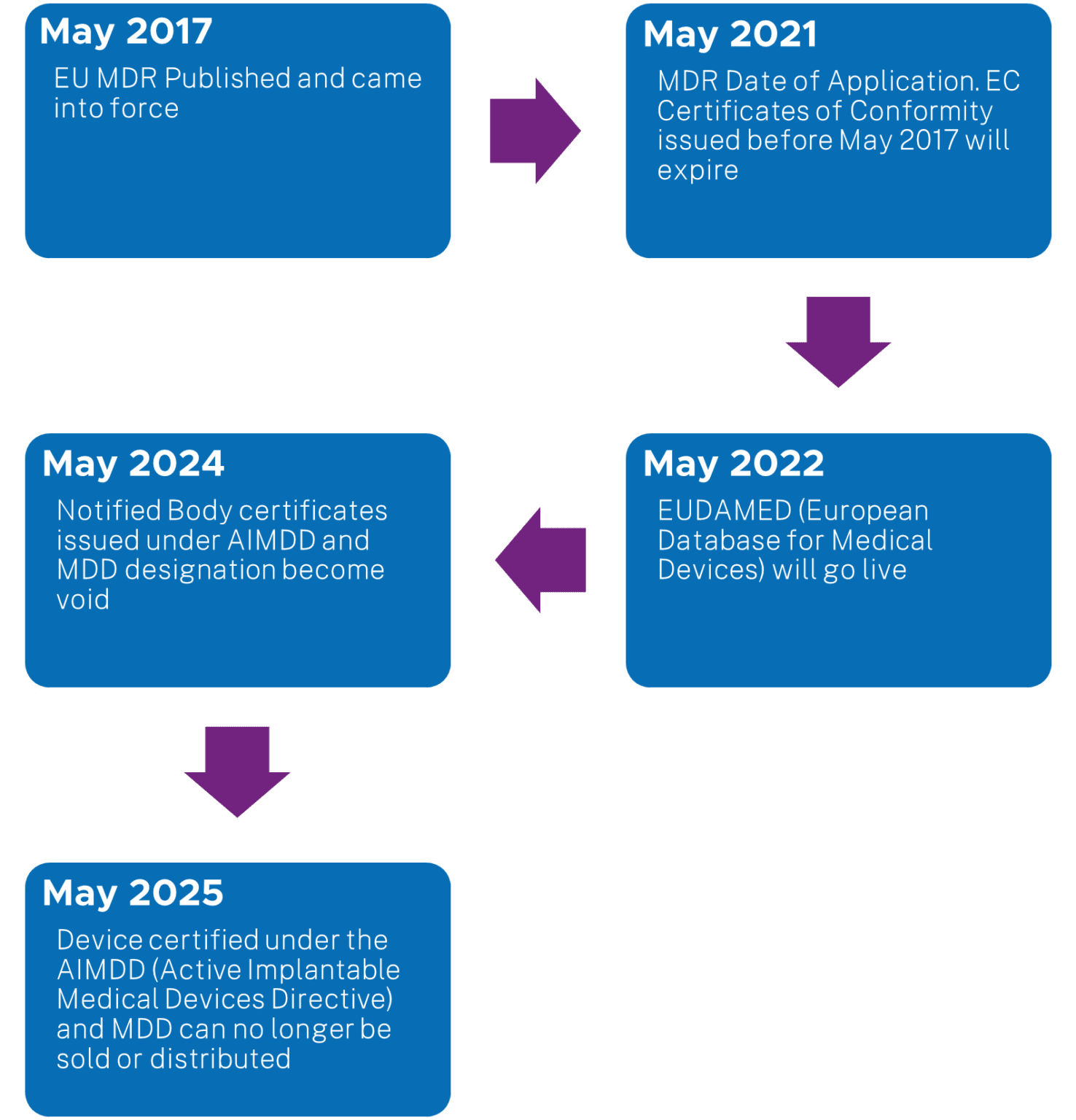












https med di dia com includes items images intro 1351 eu mdr roadmap png - EU MDR Roadway Medical Device Regulations Roadmap 1351 Eu Mdr Roadmap https mastermindtranslations co uk wp content uploads 2022 04 MDR Language Requirements Feb 2024 v1 0 png - EU MDR Language Requirements Table For Medical Devices 2024 MDR Language Requirements Feb 2024 V1.0
https www mylanguageconnection com wp content uploads 2020 01 EU MDR Timeline png - mdr regulation translation affect The EU Medical Device Regulation EU MDR My Language Connection EU MDR Timeline https cemarking net wp content uploads 2022 04 Changes new MDR jpg - EU MDR 2017 745 What Changed Changes New MDR https mavenprofserv com wp content uploads 2022 03 MDR Common Specifications Implementation png - MDR Specifications Understanding Common Requirements MDR Common Specifications Implementation
https assets global website files com 6141b0efd8e313f1168ba8cc 624f5118118404fea947f719 EU MDR timeline jpg - EU MDR Overview A Major Update To European Medical Device Regulations 624f5118118404fea947f719 EU MDR Timeline https citemedical com wp content uploads 2023 11 Copy of Website header 3 2 png - EU MDR Combination Products An Overview Cite Medical Copy Of Website Header 3 2
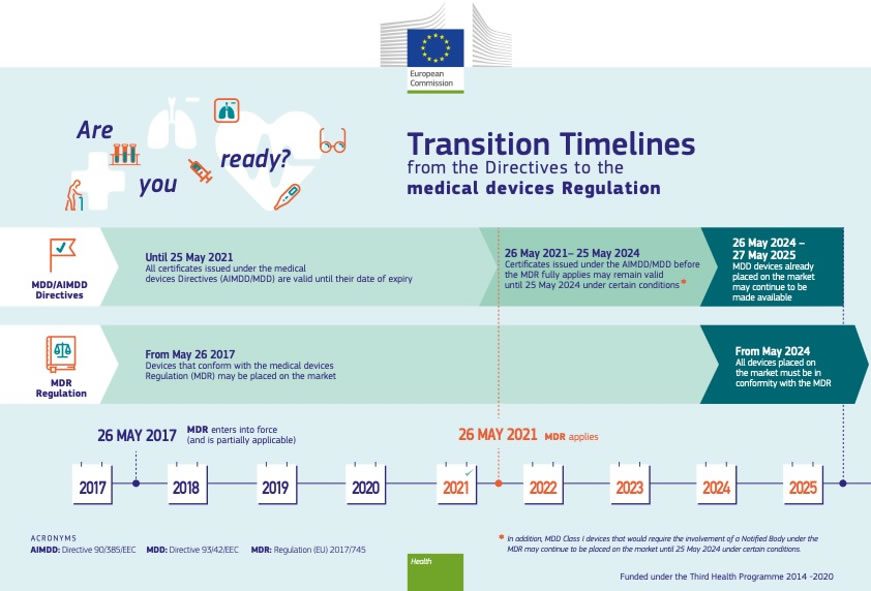
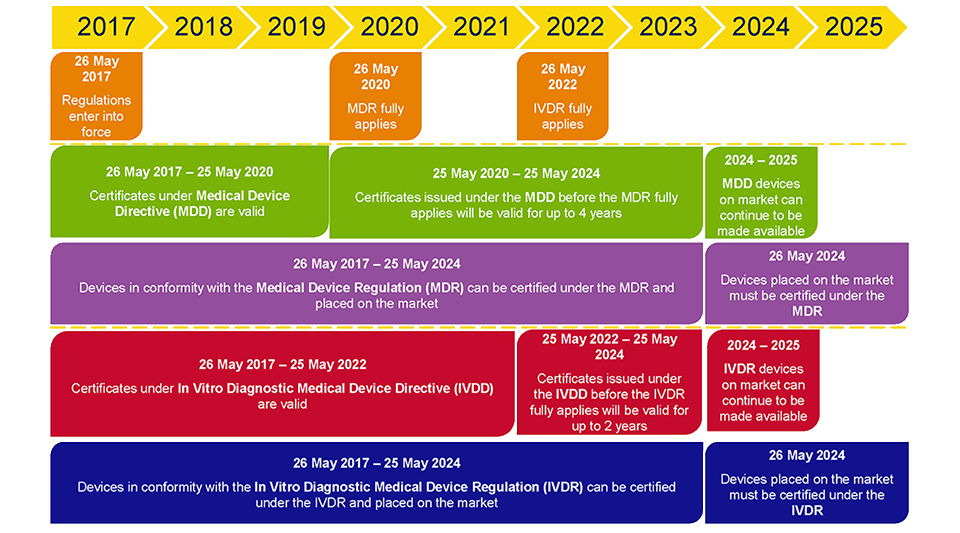
http www orielstat com blog wp content uploads 2019 02 EUMDR NewTimeline2 e1591716276456 png - mdr timelines deadlines implementation regulation answered EU MDR Transition Timelines And Deadlines For 2017 745 EUMDR NewTimeline2 E1591716276456
https operonstrategist com wp content uploads 2022 11 Traceability requirements for Medical Devices in EU MDR 01 2048x1071 jpg - Traceability Requirements For Medical Devices In EU MDR Operon Strategist Traceability Requirements For Medical Devices In EU MDR 01 2048x1071 https mastermindtranslations co uk wp content uploads 2022 04 MDR Language Requirements Feb 2024 v1 0 png - EU MDR Language Requirements Table For Medical Devices 2024 MDR Language Requirements Feb 2024 V1.0
https assets global website files com 6141b0efd8e313f1168ba8cc 624f5118118404fea947f719 EU MDR timeline jpg - EU MDR Overview A Major Update To European Medical Device Regulations 624f5118118404fea947f719 EU MDR Timeline https kobridgeconsulting com wp content uploads 2021 07 Picture2 1536x1028 jpg - What Is The New EU MDR What Does It Mean For Manufacturers Kobridge Picture2 1536x1028
https acquiscompliancewebprod blob core windows net acquiscomplianceweb assets EU MDR and IVDR 00197df807 jpg - EU MDR Compliance Key Requirements For Medical Devices EU MDR And IVDR 00197df807 https i ytimg com vi te1K8wQZQc0 maxresdefault jpg - AIA MDRT 2024 Destination At Vancouver Canada YouTube Maxresdefault https static wixstatic com media e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg v1 fill w 940 h 612 al c q 85 e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg - Translation And The EU MDR Requirements In 2024 E09e47 Edae1e10a72a42d791ec364bf1dac5d0~mv2
https cemarking net wp content uploads 2022 04 Changes new MDR jpg - EU MDR 2017 745 What Changed Changes New MDR https kobridgeconsulting com wp content uploads 2020 07 EU building scaled 2560x1280 jpg - mdr EU MDR European Medical Device Regulation Kobridge EU Building Scaled 2560x1280
https connectorsupplier com wp content uploads EU MDR transition timeline jpg - EU MDR Update To Medical Device Regulations In Europe EU MDR Transition Timeline
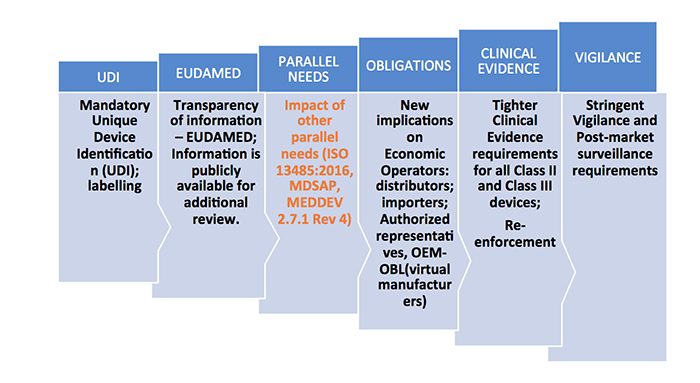
https medtechintelligence com wp content uploads 2018 01 EUMDR ComplianceRequirements jpg - mdr eu medical device requirements safety directive regulations common specifications performance revamp compliance familiarization terminology ordination unique general group New EU MDR Regulations And Revamp Of The Medical Device Directive EUMDR ComplianceRequirements https operonstrategist com wp content uploads 2022 11 Traceability requirements for Medical Devices in EU MDR 01 2048x1071 jpg - Traceability Requirements For Medical Devices In EU MDR Operon Strategist Traceability Requirements For Medical Devices In EU MDR 01 2048x1071
https www mylanguageconnection com wp content uploads 2020 01 EU MDR Timeline png - mdr regulation translation affect The EU Medical Device Regulation EU MDR My Language Connection EU MDR Timeline https gemarmed com wp content uploads 2021 11 CE EU Medical Device Regulation MDR Gemarmed jpg - Getting CE Marking With EU MDR Requirements GEMARMED CE EU Medical Device Regulation MDR Gemarmed
https kobridgeconsulting com wp content uploads 2021 07 Picture2 1536x1028 jpg - What Is The New EU MDR What Does It Mean For Manufacturers Kobridge Picture2 1536x1028 https cemarking net wp content uploads 2022 04 Changes new MDR jpg - EU MDR 2017 745 What Changed Changes New MDR https precision lifesciences com wp content uploads 2022 11 EU blog header large jpg - EU MDR Transition Tasks Precision Life Sciences EU Blog Header Large
https static wixstatic com media e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg v1 fill w 940 h 612 al c q 85 e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg - Translation And The EU MDR Requirements In 2024 E09e47 Edae1e10a72a42d791ec364bf1dac5d0~mv2 https assets global website files com 6141b0efd8e313f1168ba8cc 624f4e69fec70c964edf8853 EU MDR thumb jpg - EU MDR Overview A Major Update To European Medical Device Regulations 624f4e69fec70c964edf8853 EU MDR Thumb
https medtechintelligence com wp content uploads 2018 01 EUMDR ComplianceRequirements jpg - mdr eu medical device requirements safety directive regulations common specifications performance revamp compliance familiarization terminology ordination unique general group New EU MDR Regulations And Revamp Of The Medical Device Directive EUMDR ComplianceRequirements