Last update images today Eu Mdr Device Classification Rules













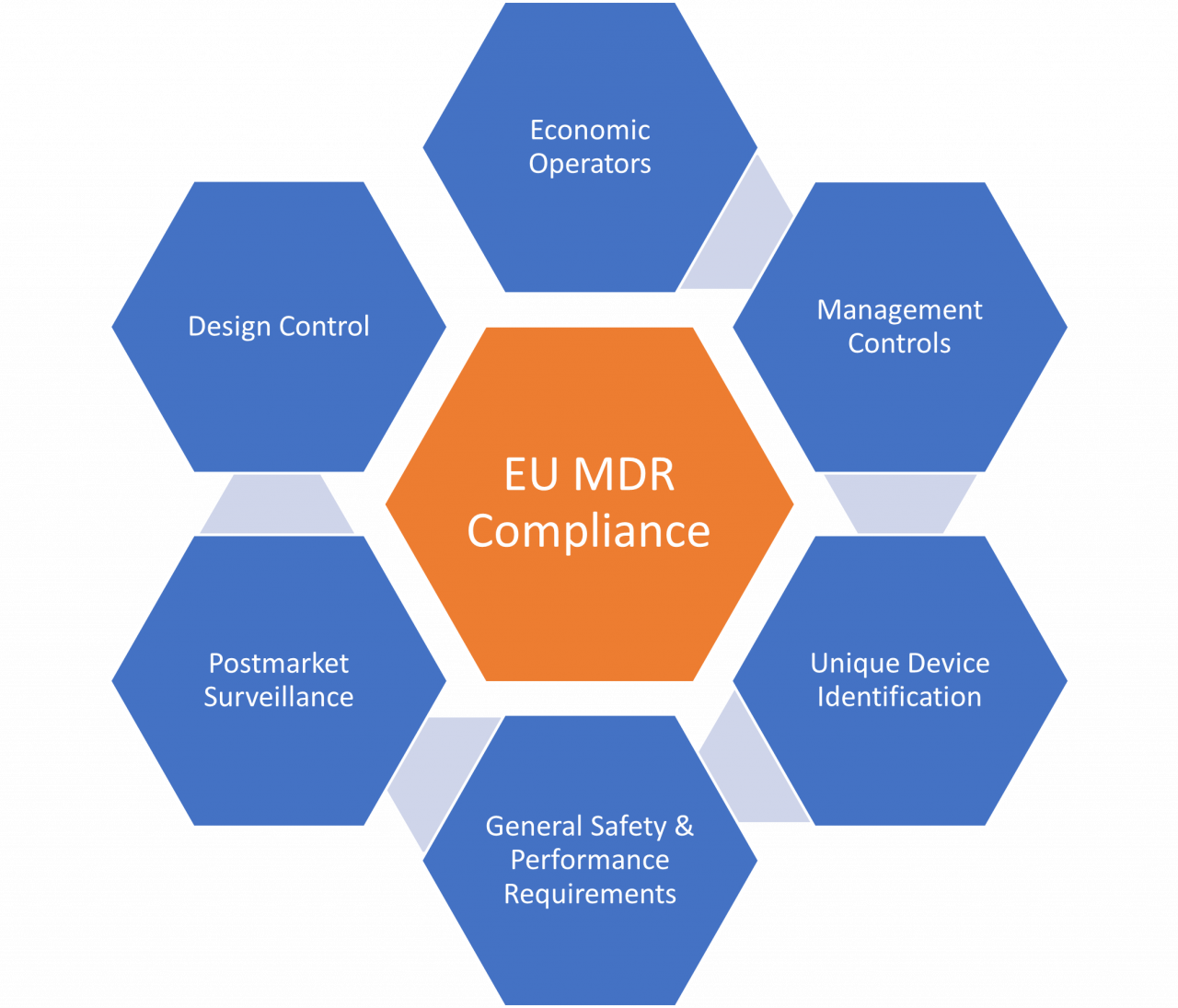




https www jamasoftware com media 2021 12 2021 12 28 new eumdr regulations 1024x512 1 jpg - Takeaways What Changes To The EU MDR Mean For You Jama Software 2021 12 28 New Eumdr Regulations 1024x512 1 https www greenlight guru hubfs Ultimate Guide to Device Class Requirements under EU MDR 1 png - Ultimate Guide To Device Class Requirements Under EU MDR Ultimate Guide To Device Class Requirements Under EU MDR 1
https operonstrategist com wp content uploads 2022 11 Traceability requirements for Medical Devices in EU MDR 01 2048x1071 jpg - Traceability Requirements For Medical Devices In EU MDR Operon Strategist Traceability Requirements For Medical Devices In EU MDR 01 2048x1071 https www i3cglobal uk wp content uploads 2022 06 Class4 jpg - Medical Device Classification EU MDR Classification Class4 https www orielstat com blog wp content uploads 2021 03 eumdr graphic e1614962121251 jpg - Understanding EU IVD Performance Evaluation Plan And Report Eumdr Graphic E1614962121251
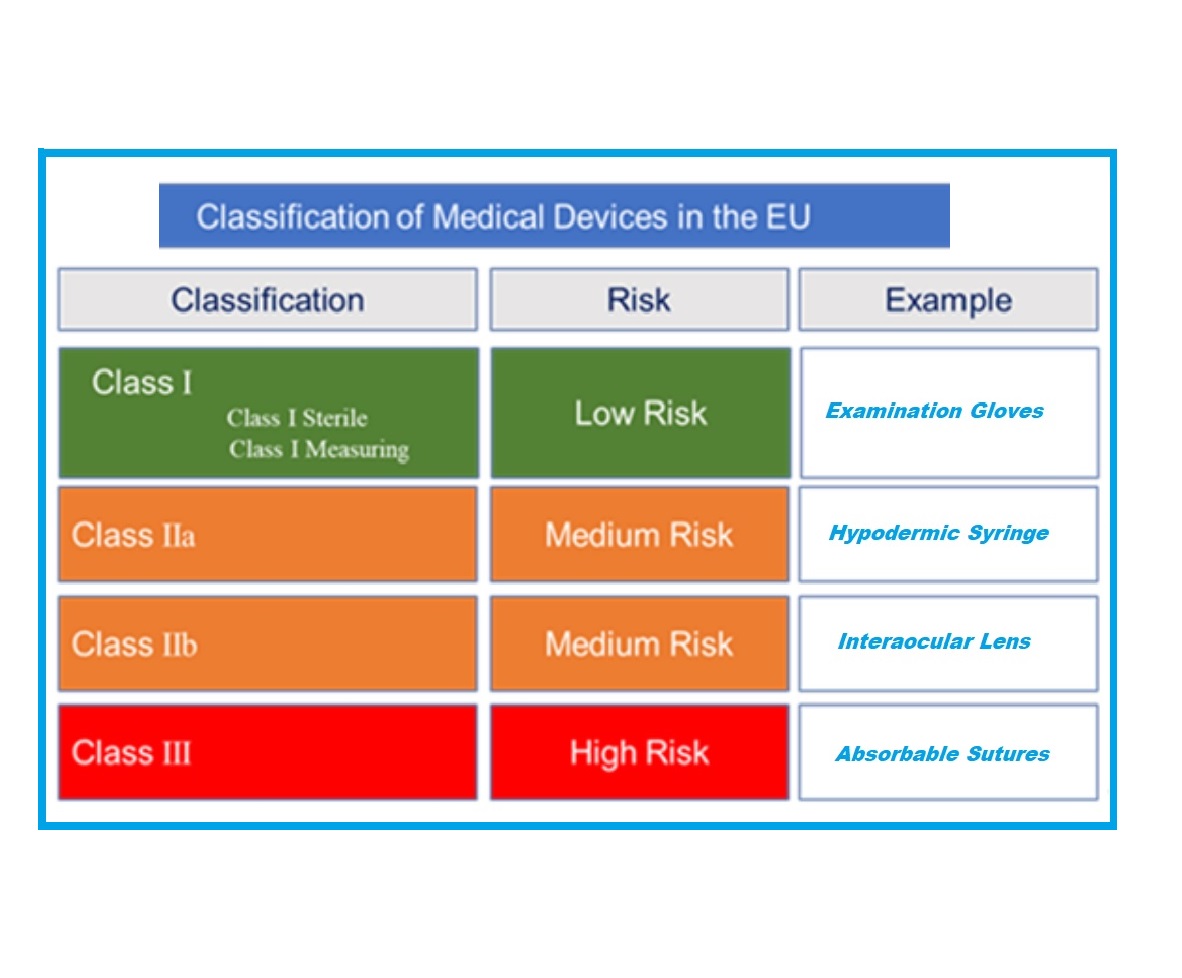
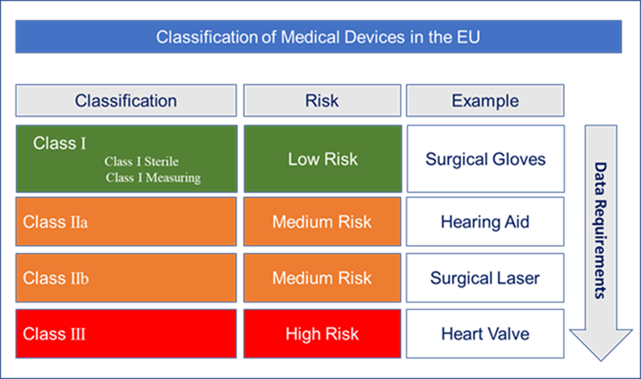
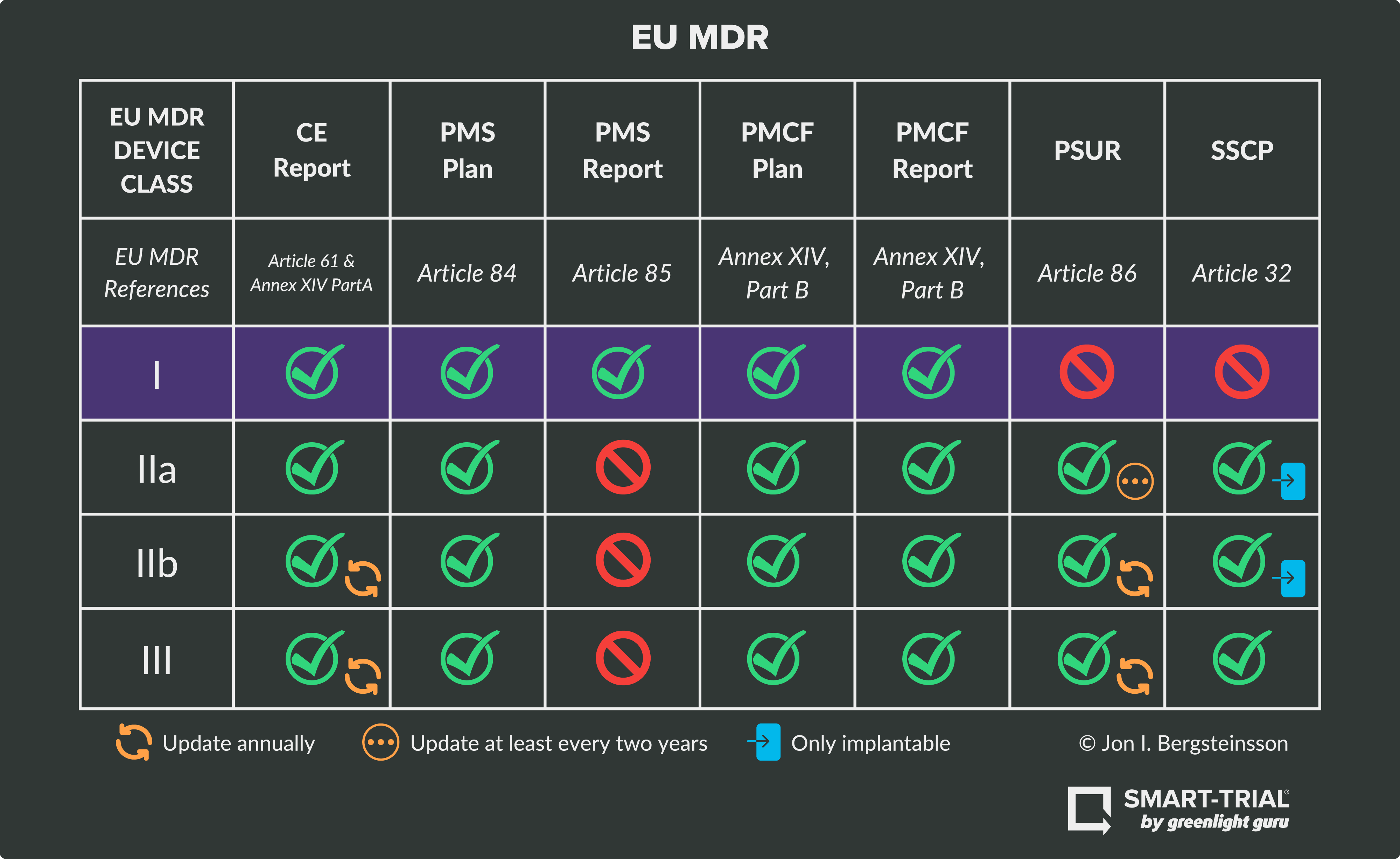
https www compliancexl com wp content uploads 2021 11 MDR Classification jpg - MDR Introduces New Rules To Classify Medical Device Classification EU MDR Classification https blog greenlight guru hubfs EU MDR Class Charts 01 png - Ultimate Guide To Device Class Requirements Under EU MDR EU MDR Class Charts 01
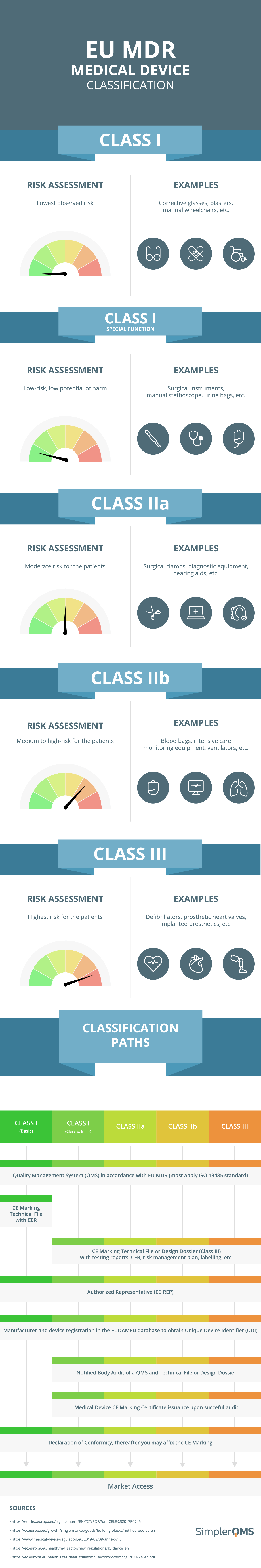
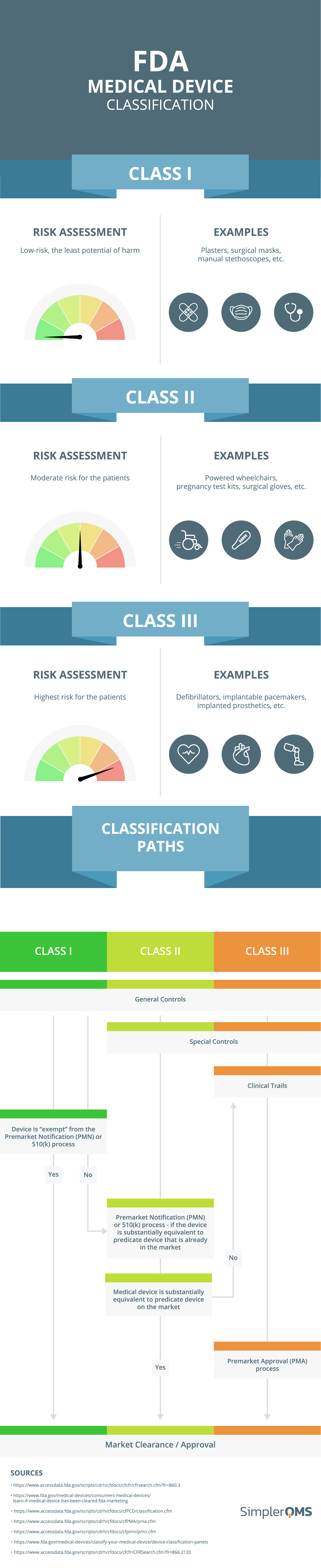
https www simplerqms com wp content uploads 2021 11 eu mdr medical device classification infographic png - Medical Device Classification FDA EU MDR SimplerQMS 2024 Eu Mdr Medical Device Classification Infographic
https vertassets blob core windows net image 9d33fdde 9d33fdde fcf2 4cf1 89c2 91b0d38ad7f1 device type jpg - EU MDR Now Regulates Beauty Devices As Medical Devices Device Type https www ppd com wp content uploads 2021 05 Classification Medical Devices EU png - Approaching MDR Compliance PPD Inc Classification Medical Devices EU
https www orielstat com blog wp content uploads 2021 03 eumdr graphic e1614962121251 jpg - Understanding EU IVD Performance Evaluation Plan And Report Eumdr Graphic E1614962121251 https learningcenter sourceintelligence com hs fs hubfs Copy of Copy of EU IVDR and MDR Classification jpg - Mdr Classification Chart Copy Of Copy Of EU IVDR And MDR Classification
https spyro soft com wp content uploads 2022 08 spyrosoft wykres 18 02 2022 ver01 1024x560 jpg - The Complete Guide To EU Medical Device Regulation Spyrosoft Spyrosoft Wykres 18.02.2022 Ver01 1024x560 https medicaldeviceslegal files wordpress com 2023 03 mdr amendment flowchart3 jpg - MDR And IVDR Amendment Has Entered Into Force Now Medicaldeviceslegal Mdr Amendment Flowchart3 https advisera com wp content uploads sites 14 2021 04 what are eu mdr classification rules for medical devices 768x317 png - mdr devices rules EU MDR Device Classification Guide Advisera What Are Eu Mdr Classification Rules For Medical Devices 768x317
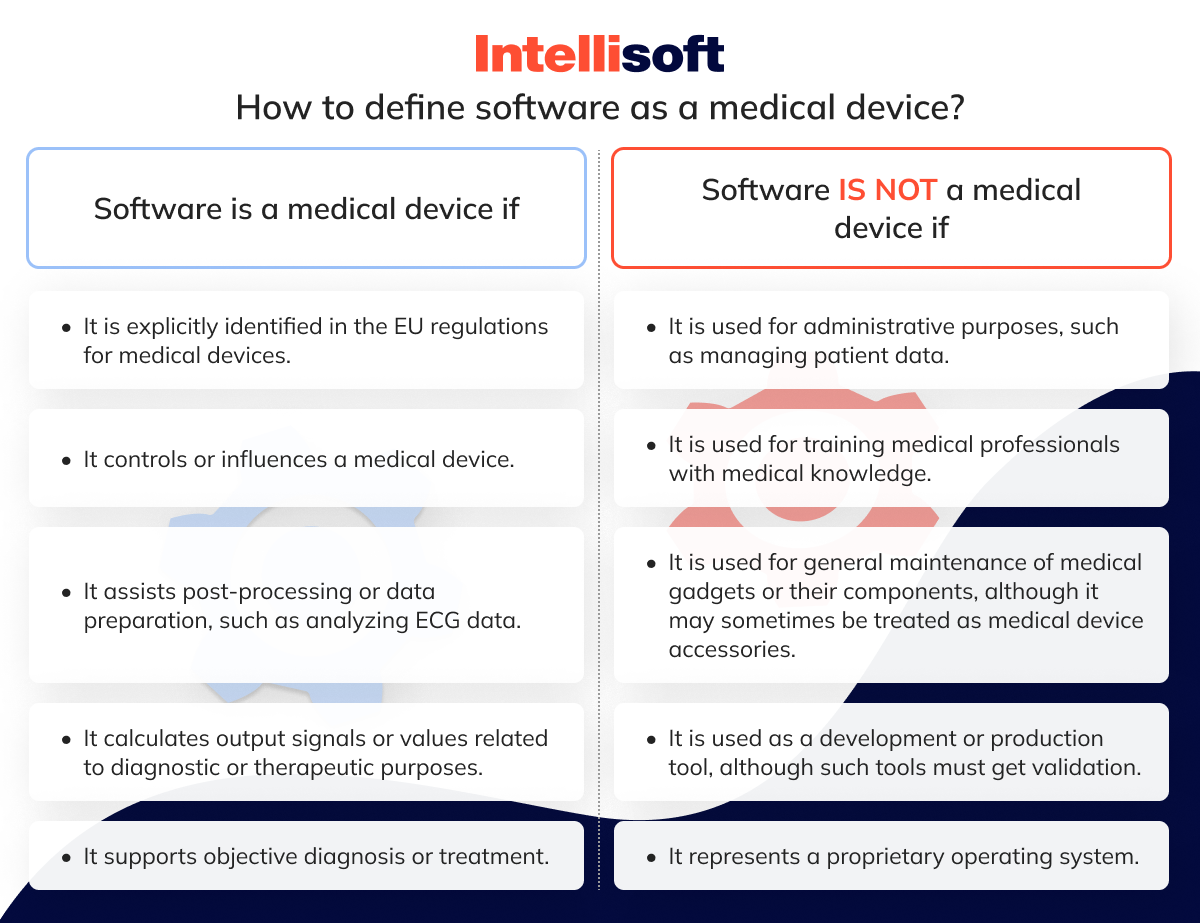
https www ideagen com media 11414 id social understanding the eu mdr classifaction rules 01 png - Mdr Medical Device Classifications MeaningKosh Id Social Understanding The Eu Mdr Classifaction Rules 01 https platohealth ai wp content uploads 2023 09 ultimate guide to device class requirements under eu mdr 1 png - Ultimate Guide To Device Class Requirements Under EU MDR PlatoHealth Ultimate Guide To Device Class Requirements Under Eu Mdr 1
https qbdgroup com wp content uploads What Does the MDR transition Mean for Your Medical Device Flowchart QbD Group V02 png - How To Plan MDR Compliance For Your Medical Device What Does The MDR Transition Mean For Your Medical Device Flowchart QbD Group V02
https healthwavehub com api download qr - EU MDR Device Classification Qrhttp ce marking org images mdd classification chart active gif - Mdr Device Classification Flowchart Chart Examples Mdd Classification Chart Active
https www ideagen com media 11414 id social understanding the eu mdr classifaction rules 01 png - Mdr Medical Device Classifications MeaningKosh Id Social Understanding The Eu Mdr Classifaction Rules 01 https www i3cglobal uk wp content uploads 2022 06 Class4 jpg - Medical Device Classification EU MDR Classification Class4
https static wixstatic com media e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg v1 fill w 940 h 612 al c q 85 e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg - Translation And The EU MDR Requirements In 2024 E09e47 Edae1e10a72a42d791ec364bf1dac5d0~mv2 https www ppd com wp content uploads 2021 05 Classification Medical Devices EU png - Approaching MDR Compliance PPD Inc Classification Medical Devices EU https operonstrategist com wp content uploads 2022 11 Traceability requirements for Medical Devices in EU MDR 01 2048x1071 jpg - Traceability Requirements For Medical Devices In EU MDR Operon Strategist Traceability Requirements For Medical Devices In EU MDR 01 2048x1071
https www avanti europe ch wp content uploads 2020 09 MDR classification rules png - mdr classification rules under What You Need To Know About The MDR Classification Rules MDR Classification Rules https www regdesk co wp content uploads 2023 04 EU MDR Overview 1280x1095 png - EU MDR Overview An Update To European Medical Device Regulations RegDesk EU MDR Overview 1280x1095
http ce marking org images mdd classification chart active gif - Mdr Device Classification Flowchart Chart Examples Mdd Classification Chart Active