Last update images today Eu Mdr Investigational Devices Definition
























https intellisoft io wp content uploads 2024 03 4 20 png - EU Medical Device Regulation Compliance And Updates In 2024 4 20 https www arenasolutions com wp content uploads what is medical device regulation png - Medical Device Regulation Definition Arena What Is Medical Device Regulation
https www shutterstock com shutterstock photos 1927025528 display 1500 stock vector mdr medical device regulation regulation of the eu european union on the clinical investigation 1927025528 jpg - 11 Life Sciences Mdr Images Stock Photos Vectors Shutterstock Stock Vector Mdr Medical Device Regulation Regulation Of The Eu European Union On The Clinical Investigation 1927025528 https assets website files com 5ebe569becbcbc7848cc9c42 608aaf5f7b4108d4cfe4e6dc mdcg 2021 6 en png - New Guidance On How To Conduct Clinical Investigations Under The EU MDR 608aaf5f7b4108d4cfe4e6dc Mdcg 2021 6 En https operonstrategist com wp content uploads 2022 11 Traceability requirements for Medical Devices in EU MDR 01 1024x535 jpg - Traceability Requirements For Medical Devices In EU MDR Operon Strategist Traceability Requirements For Medical Devices In EU MDR 01 1024x535
https gemarmed com wp content uploads 2021 11 CE EU Medical Device Regulation MDR Gemarmed jpg - Getting CE Marking With EU MDR Requirements GEMARMED CE EU Medical Device Regulation MDR Gemarmed https www ophthalmologybreakingnews com files images blog european parliament extends mdr compliance timeline to 2027 2028 jpg - European Parliament Extends MDR Transition To 2027 2028 OBN Blog European Parliament Extends Mdr Compliance Timeline To 2027 2028
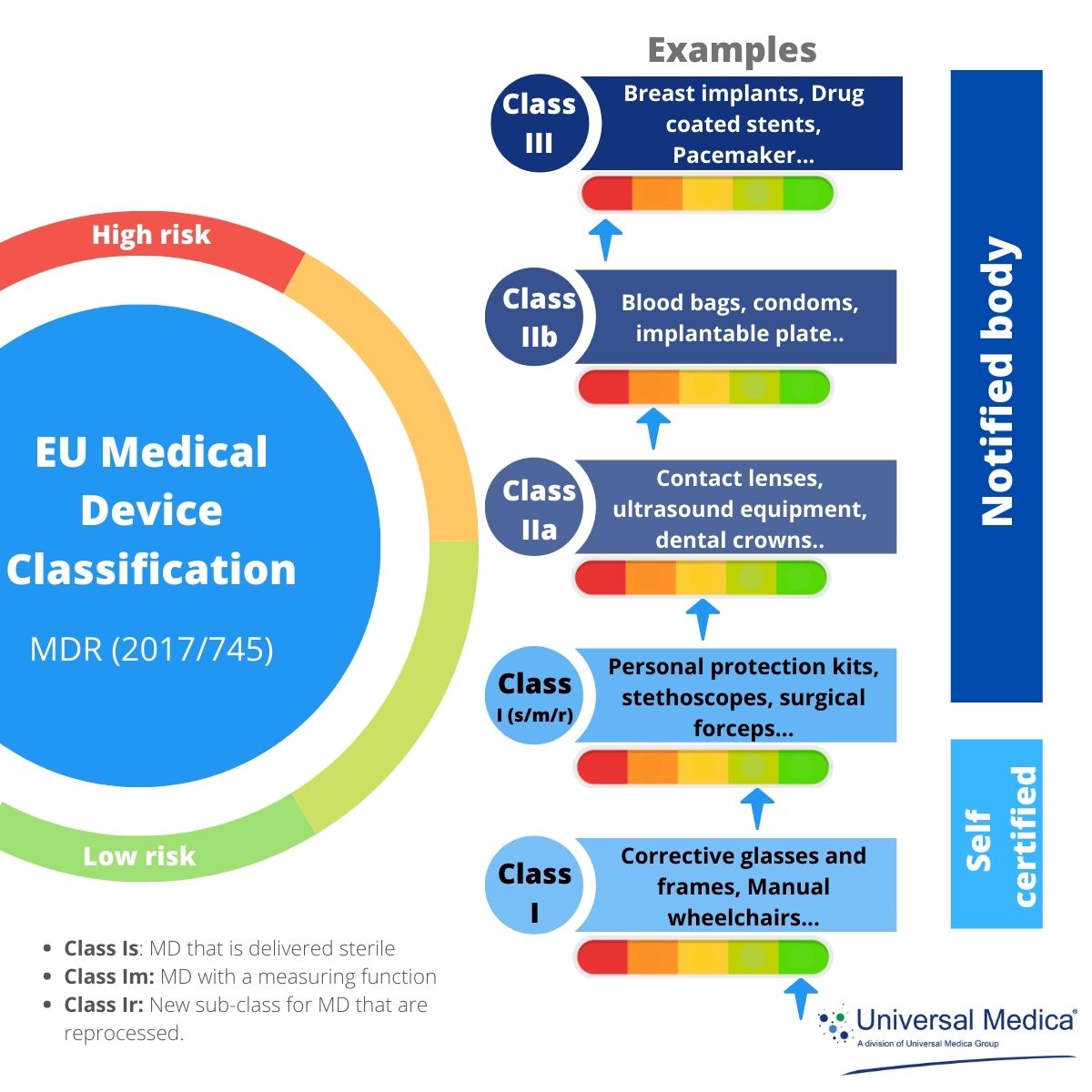
https www arenasolutions com wp content uploads MDR IVDR Device Classifications Feature image 768x768 jpg - How To Classify Your Medical Device Under The EU MDR And IVDR Arena MDR IVDR Device Classifications Feature Image 768x768
https www ophthalmologybreakingnews com files images blog european parliament extends mdr compliance timeline to 2027 2028 jpg - European Parliament Extends MDR Transition To 2027 2028 OBN Blog European Parliament Extends Mdr Compliance Timeline To 2027 2028 https static wixstatic com media 78a52e 77d1ade220e94d6aa586d788f9c6d1d4 mv2 png v1 fill w 940 h 612 al c q 90 78a52e 77d1ade220e94d6aa586d788f9c6d1d4 mv2 png - Understanding The New EU MDR Update And Its Effect On Medical Device 78a52e 77d1ade220e94d6aa586d788f9c6d1d4~mv2
https www ophthalmologybreakingnews com files images blog european parliament extends mdr compliance timeline to 2027 2028 jpg - European Parliament Extends MDR Transition To 2027 2028 OBN Blog European Parliament Extends Mdr Compliance Timeline To 2027 2028 https assets website files com 5ebe569becbcbc7848cc9c42 608aa77a36463ec4a1c48c43 New Guidance on How to Conduct Clinical Investigations Under the EU MDR png - New Guidance On How To Conduct Clinical Investigations Under The EU MDR 608aa77a36463ec4a1c48c43 New Guidance On How To Conduct Clinical Investigations Under The EU MDR
https ds982gbwnvyso cloudfront net Pictures web t u j eumdrupdtfig2 800235 svgz - EU MDR Implementation What Is Changing For The Medical Device Eumdrupdtfig2 800235.svgzhttps rp cro com static RP CRO MDR case study jpg - Accelerating Submissions Under New EU Medical Device Regulations MDR RP CRO MDR Case Study https www medialocate com wp content uploads EU MDR Translation Requirements MediaLocate jpg - EU MDR Medical Device Regulation Translation Requirements MediaLocate EU MDR Translation Requirements MediaLocate
https www arenasolutions com wp content uploads MDR IVDR Device Classifications Feature image 768x768 jpg - How To Classify Your Medical Device Under The EU MDR And IVDR Arena MDR IVDR Device Classifications Feature Image 768x768 https assets global website files com 6141b0efd8e313f1168ba8cc 624f5118118404fea947f719 EU MDR timeline jpg - EU MDR Overview A Major Update To European Medical Device Regulations 624f5118118404fea947f719 EU MDR Timeline
https www fdanews com ext resources Book Covers NEW Complying with the EU MDR 500 jpg - Complying With The EU MDR New Guidances Standards And Procedures Complying With The EU MDR 500
https static wixstatic com media 78a52e 77d1ade220e94d6aa586d788f9c6d1d4 mv2 png v1 fill w 940 h 612 al c q 90 78a52e 77d1ade220e94d6aa586d788f9c6d1d4 mv2 png - Understanding The New EU MDR Update And Its Effect On Medical Device 78a52e 77d1ade220e94d6aa586d788f9c6d1d4~mv2 https www simplerqms com wp content uploads 2024 01 eu mdr medical device approval process svg - EU MDR Medical Device Classification Classes And Examples Eu Mdr Medical Device Approval Process.svg
https static wixstatic com media ec71d0 b6fe9410ee254377906af801da73f9fe mv2 png v1 fill w 692 h 496 al c q 85 enc auto ec71d0 b6fe9410ee254377906af801da73f9fe mv2 png - Regulation Of Reusable Medical Devices Under EU MDR Ec71d0 B6fe9410ee254377906af801da73f9fe~mv2 https rp cro com static RP CRO MDR case study jpg - Accelerating Submissions Under New EU Medical Device Regulations MDR RP CRO MDR Case Study
https operonstrategist com wp content uploads 2022 11 Traceability requirements for Medical Devices in EU MDR 01 1024x535 jpg - Traceability Requirements For Medical Devices In EU MDR Operon Strategist Traceability Requirements For Medical Devices In EU MDR 01 1024x535 https www onlinegmptraining com wp content uploads 2022 10 EU MDR downloadPDF 674x405 png - EU MDR EU Medical Device Regulation IVDR Compliance EU MDR DownloadPDF 674x405 https learngxp com wp content uploads 2020 07 317 318 319 320 321 322 326 327 328 2 png - EU Medical Device Regulation EU MDR Chapter 3 Identification 317 318 319 320 321 322 326 327 328 2
https static wixstatic com media e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg v1 fill w 940 h 612 al c q 85 e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg - Translation And The EU MDR Requirements In 2024 E09e47 Edae1e10a72a42d791ec364bf1dac5d0~mv2 https assets global website files com 6141b0efd8e313f1168ba8cc 624f5118118404fea947f719 EU MDR timeline jpg - EU MDR Overview A Major Update To European Medical Device Regulations 624f5118118404fea947f719 EU MDR Timeline
https assets website files com 5ebe569becbcbc7848cc9c42 608aaf5f7b4108d4cfe4e6dc mdcg 2021 6 en png - New Guidance On How To Conduct Clinical Investigations Under The EU MDR 608aaf5f7b4108d4cfe4e6dc Mdcg 2021 6 En