Last update images today Eu Mdr Investigational Devicesdefinition
























https acquiscompliancewebprod blob core windows net acquiscomplianceweb assets EU MDR and IVDR 00197df807 jpg - EU MDR Compliance Key Requirements For Medical Devices EU MDR And IVDR 00197df807 https operonstrategist com wp content uploads 2022 11 Traceability requirements for Medical Devices in EU MDR 01 1024x535 jpg - Traceability Requirements For Medical Devices In EU MDR Operon Strategist Traceability Requirements For Medical Devices In EU MDR 01 1024x535
https www eucope org wp content uploads 2023 01 news posts 1024x576 png - MDR IVDR Implementation EC S Proposal On 6 January 2023 News Posts 1024x576 https cleanroomconnect com wp content uploads 2019 05 EU MDR jpg - mdr eu EU MDR The European Union Medical Device Regulation Of 2017 EU MDR https i pinimg com originals b0 15 62 b01562792b2efcde927a3d9089bdcb7d jpg - How To Ensure Compliance With The New EU Medical Device Regulations B01562792b2efcde927a3d9089bdcb7d
https static wixstatic com media e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg v1 fill w 940 h 612 al c q 85 e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg - Translation And The EU MDR Requirements In 2024 E09e47 Edae1e10a72a42d791ec364bf1dac5d0~mv2 https www sofeast com wp content uploads 2021 10 EU MDR Compliance When Developing A New Medical Device High Level Steps Guide jpeg - EU MDR Compliance When Developing A New Medical Device In China High EU MDR Compliance When Developing A New Medical Device High Level Steps Guide
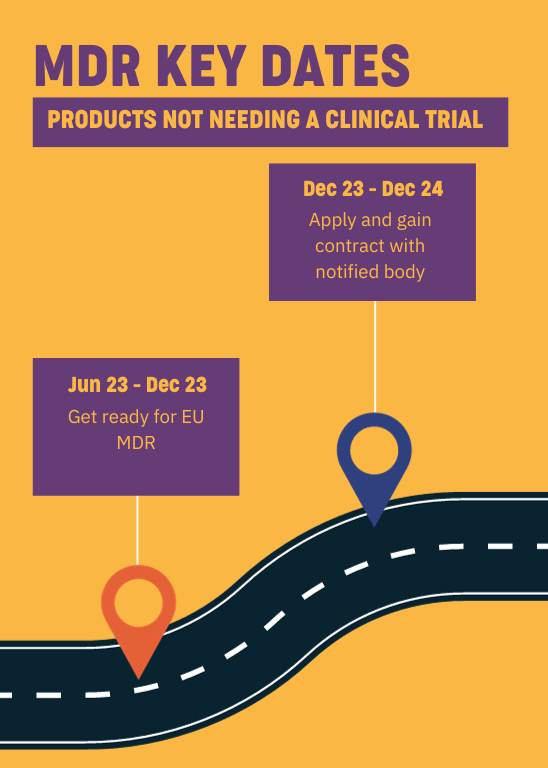
http www orielstat com blog wp content uploads 2018 07 EU MDR Timeline 2 png - mdr eu transition timeline medical device regulation mdd timelines changes directive difference ce overview marking compliance Preparing For The EU MDR 2020 Changes Oriel STAT A MATRIX EU MDR Timeline 2 https www innovit com wp content uploads 2021 09 Intersection 7 png - Overview Of The European Databank On Medical Devices MDR Innovit Intersection 7
https assets global website files com 6141b0efd8e313f1168ba8cc 624f5118118404fea947f719 EU MDR timeline jpg - EU MDR Overview A Major Update To European Medical Device Regulations 624f5118118404fea947f719 EU MDR Timeline https connectorsupplier com wp content uploads EU MDR transition timeline 600x407 jpg - EU MDR Update To Medical Device Regulations In Europe EU MDR Transition Timeline 600x407
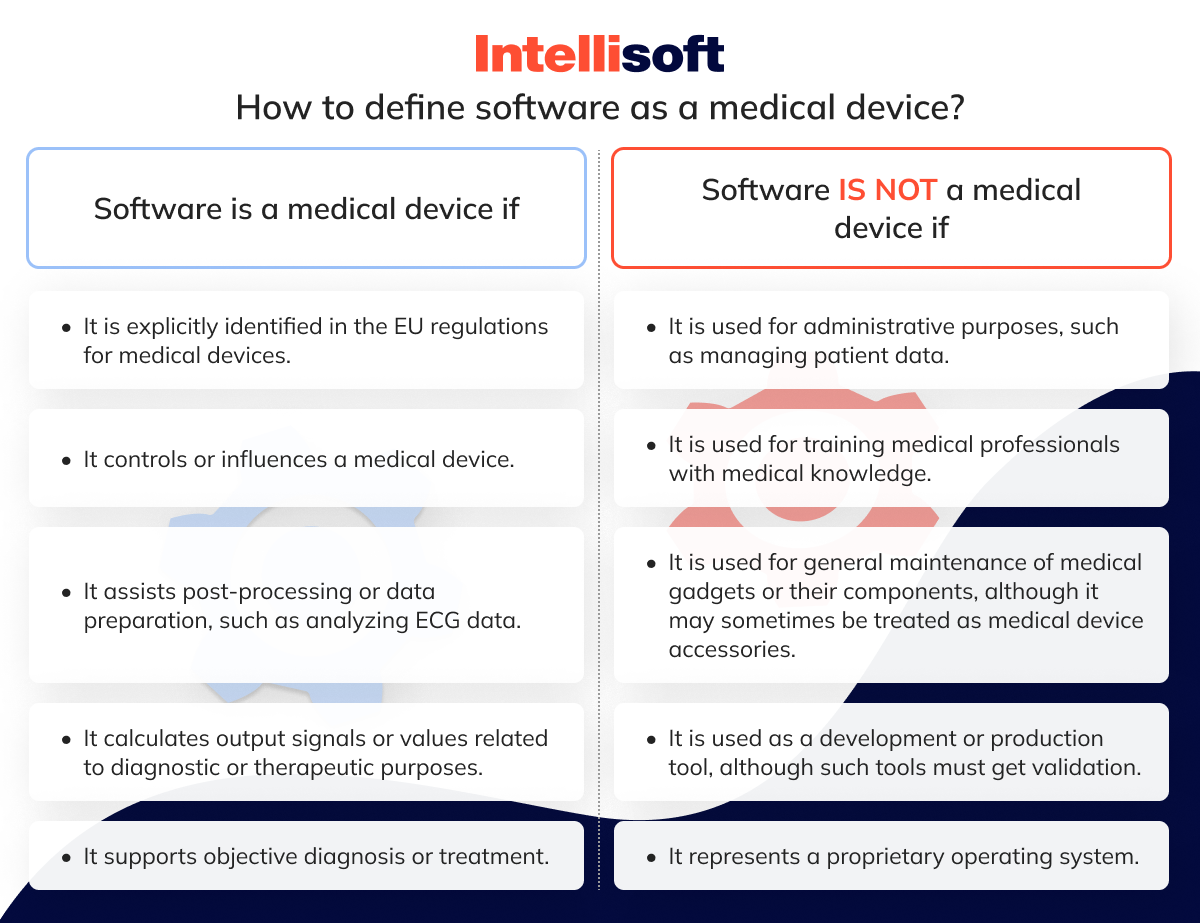
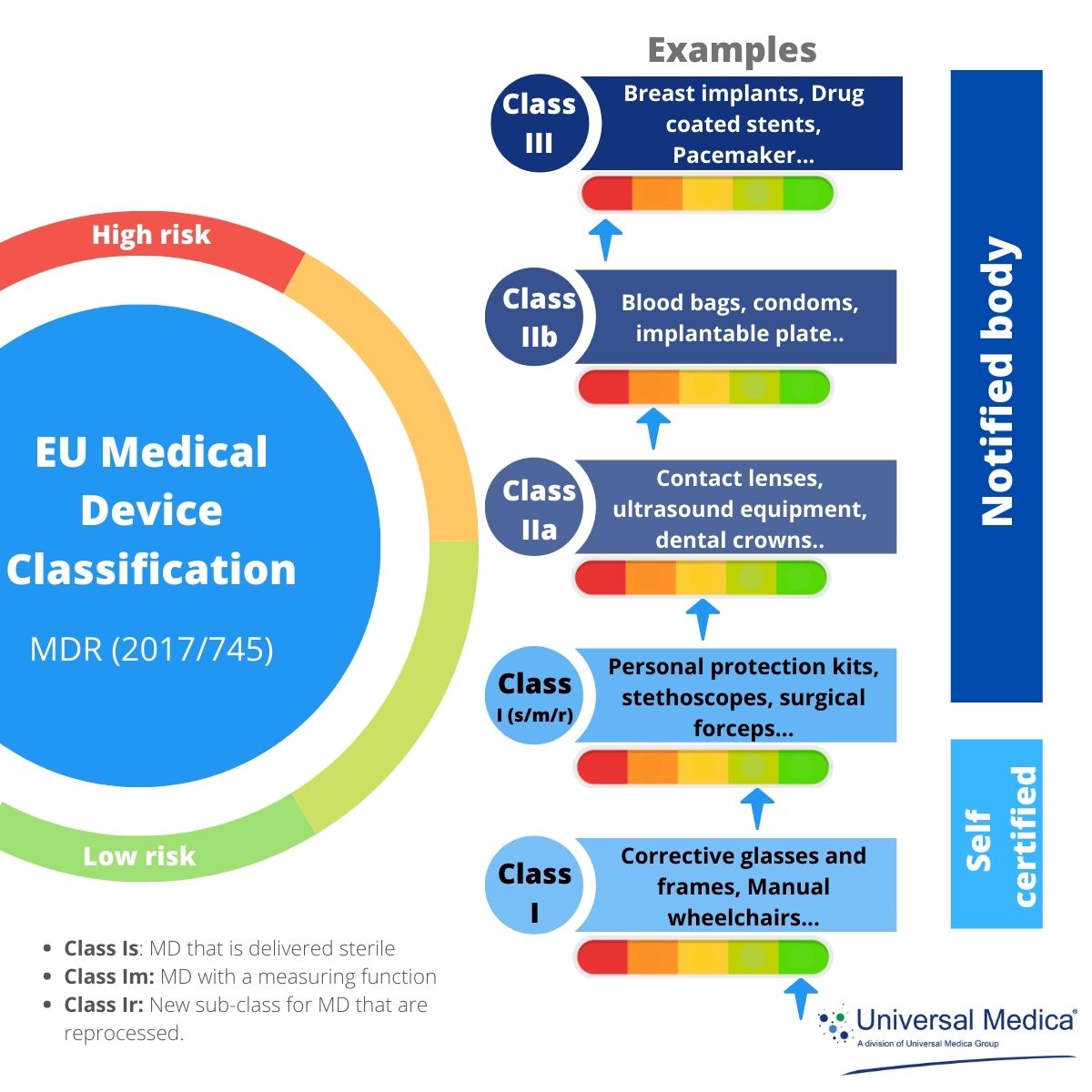
https static wixstatic com media e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg v1 fill w 940 h 612 al c q 85 e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg - Translation And The EU MDR Requirements In 2024 E09e47 Edae1e10a72a42d791ec364bf1dac5d0~mv2 https www simplerqms com wp content uploads 2024 01 eu mdr medical device approval process svg - EU MDR Medical Device Classification Classes And Examples Eu Mdr Medical Device Approval Process.svghttps assets website files com 5ebe569becbcbc7848cc9c42 608aaf5f7b4108d4cfe4e6dc mdcg 2021 6 en png - New Guidance On How To Conduct Clinical Investigations Under The EU MDR 608aaf5f7b4108d4cfe4e6dc Mdcg 2021 6 En
https www fdanews com ext resources Book Covers NEW Complying with the EU MDR 500 jpg - Complying With The EU MDR New Guidances Standards And Procedures Complying With The EU MDR 500 https acquiscompliancewebprod blob core windows net acquiscomplianceweb assets EU MDR and IVDR 00197df807 jpg - EU MDR Compliance Key Requirements For Medical Devices EU MDR And IVDR 00197df807
https rp cro com static RP CRO MDR case study jpg - Accelerating Submissions Under New EU Medical Device Regulations MDR RP CRO MDR Case Study https www innovit com wp content uploads 2021 09 Intersection 7 png - Overview Of The European Databank On Medical Devices MDR Innovit Intersection 7
https www fticonsulting com en uk insights articles media 36573f57b11c436fa3e383c0144dd4ac ashx - The Medical Device Regulation MDR FTI Consulting 36573f57b11c436fa3e383c0144dd4ac.ashxhttps www innovit com wp content uploads 2021 09 Intersection 7 png - Overview Of The European Databank On Medical Devices MDR Innovit Intersection 7
https www medialocate com wp content uploads EU MDR Translation Requirements MediaLocate jpg - EU MDR Medical Device Regulation Translation Requirements MediaLocate EU MDR Translation Requirements MediaLocate http www orielstat com blog wp content uploads 2018 07 EU MDR Timeline 2 png - mdr eu transition timeline medical device regulation mdd timelines changes directive difference ce overview marking compliance Preparing For The EU MDR 2020 Changes Oriel STAT A MATRIX EU MDR Timeline 2 https i pinimg com originals b0 15 62 b01562792b2efcde927a3d9089bdcb7d jpg - How To Ensure Compliance With The New EU Medical Device Regulations B01562792b2efcde927a3d9089bdcb7d
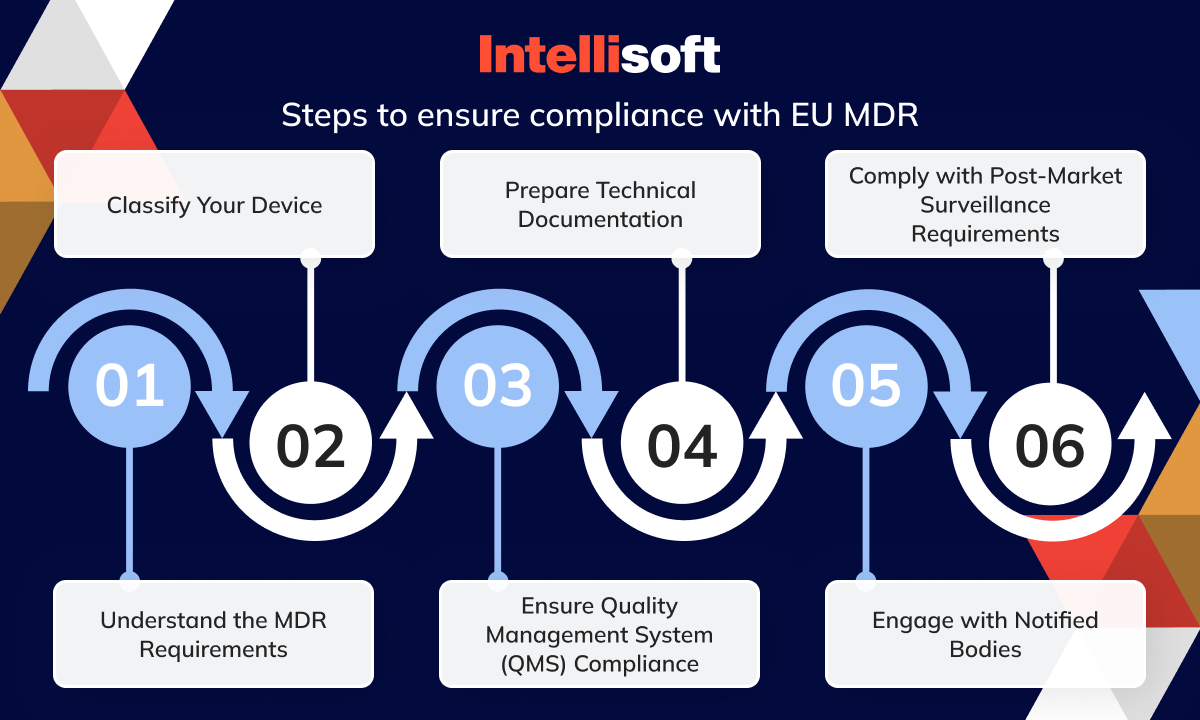
https intellisoft io wp content uploads 2024 03 4 20 png - EU Medical Device Regulation Compliance And Updates In 2024 4 20 https www onlinegmptraining com wp content uploads 2022 10 EUMDR medicaldeviceregulation download jpg - EU MDR EU Medical Device Regulation IVDR Compliance EUMDR Medicaldeviceregulation Download