Last update images today Eu Mdr Regulation Eu 2017 745
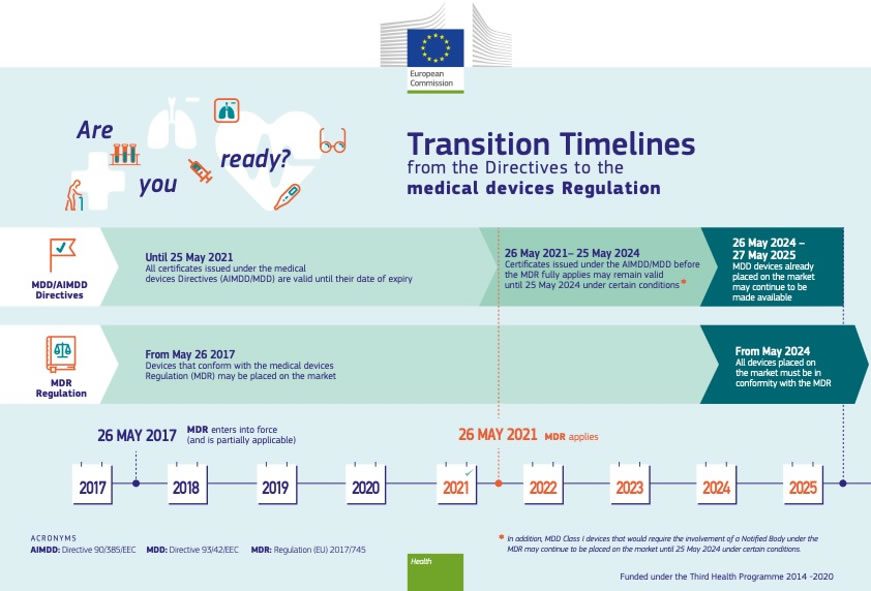












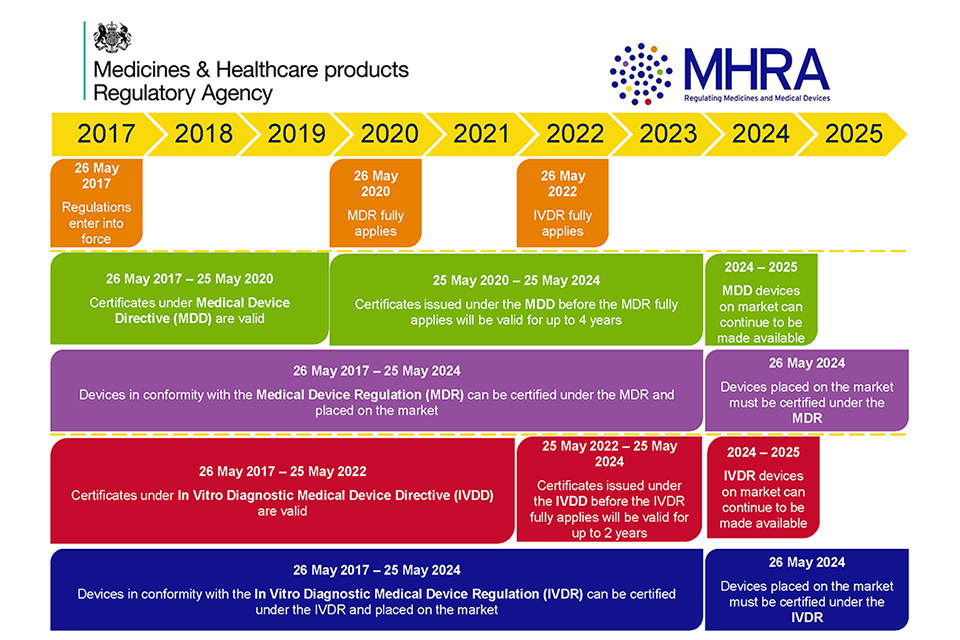










https assets publishing service gov uk government uploads system uploads image data file 66243 MHRA Certificate Validity 960x640 png - medical mdr ivdr devices eu regulations period ce certificate directives gov mhra under date transition guidance four after file issued Medical Devices EU Regulations For MDR And IVDR GOV UK MHRA Certificate Validity 960x640 https gxp training com wp content uploads 2023 06 MDR 2017 png - Medical Device Regulation MDR 2017 745 Course And Certificate MDR 2017
https cdn01 alison static net courses 2218 alison courseware intro 2218 jpg - mdr eu regulations device medical alison essentials european EU Medical Device Regulations 2017 745 Free Online Course Alison Alison Courseware Intro 2218 https i1 rgstatic net publication 322525958 The New European Medical Device Regulation 2017745 Main Changes and Challenges links 5a5e035ea6fdcc68fa990858 largepreview png - regulation medical device european challenges changes main pdf PDF The New European Medical Device Regulation 2017 745 Main Changes Largepreview https info qbdgroup com hs fs hubfs QD200503 WP MDR 2017745 DEF 1 jpg - MDR 2017 745 QD200503 WP MDR 2017745 DEF 1
https www orielstat com blog wp content uploads 2018 08 CE Mark 300x200 png - Preparing For The EU MDR 2020 Changes Oriel STAT A MATRIX CE Mark 300x200 https focus pqegroup com hs fs hubfs MDR book 1 png - Get Compliant With EU MDR 2017 745 MDR Book 1
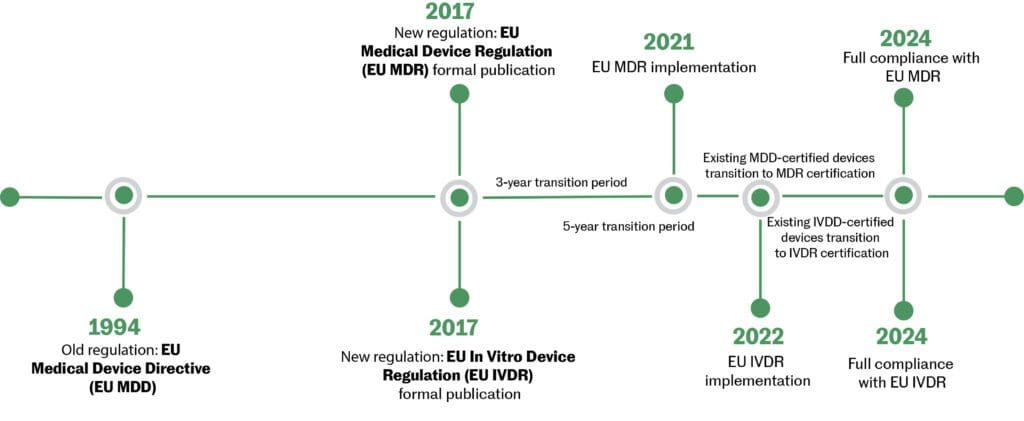
https medtechintelligence com wp content uploads 2018 01 EUMDR TransitionTimeline jpg - mdr eu medical device directive regulations classification revamp transition events timelines article New EU MDR Regulations And Revamp Of The Medical Device Directive EUMDR TransitionTimeline
https karevcert com wp content uploads 2021 10 eumdr jpg - MDR 2017 745 KAREV Eumdr https info qbdgroup com hs fs hubfs QD200503 WP MDR 2017745 DEF 1 jpg - MDR 2017 745 QD200503 WP MDR 2017745 DEF 1
https karevcert com wp content uploads 2021 10 eumdr jpg - MDR 2017 745 KAREV Eumdr https www orielstat com blog wp content uploads 2018 08 CE Mark 300x200 png - Preparing For The EU MDR 2020 Changes Oriel STAT A MATRIX CE Mark 300x200
https medtechintelligence com wp content uploads 2018 01 EUMDR TransitionTimeline jpg - mdr eu medical device directive regulations classification revamp transition events timelines article New EU MDR Regulations And Revamp Of The Medical Device Directive EUMDR TransitionTimeline https focus pqegroup com hs fs hubfs MDR book 1 png - Get Compliant With EU MDR 2017 745 MDR Book 1 https www presentationeze com wp content uploads MDR Medical Device Regulation EU 2017 745 Timeline jpg - mdr eu medical device timeline regulation MDR Medical Device Regulation EU 2017 745 Timeline PresentationEZE MDR Medical Device Regulation EU 2017 745 Timeline
https www ophthalmologybreakingnews com files images blog european parliament extends mdr compliance timeline to 2027 2028 jpg - European Parliament Extends MDR Transition To 2027 2028 OBN Blog European Parliament Extends Mdr Compliance Timeline To 2027 2028 http www orielstat com blog wp content uploads 2019 02 EUMDR NewTimeline2 e1591716276456 png - mdr timelines deadlines implementation regulation answered EU MDR Transition Timelines And Deadlines For 2017 745 EUMDR NewTimeline2 E1591716276456
https www bayoocare com byn uploads sites 10 2022 10 MDR Zertifikat Canva v2 png - MDR Certificate For Class IIb Medical Device BAYOOCARE MDR Zertifikat Canva V2
https www arenasolutions com wp content uploads what is MDR 2017 745 Compliance png - MDR 2017 745 Compliance Definition Arena What Is MDR 2017 745 Compliance https info qbdgroup com hs fs hubfs QD200503 WP MDR 2017745 DEF 1 jpg - MDR 2017 745 QD200503 WP MDR 2017745 DEF 1
https www ophthalmologybreakingnews com files images blog european parliament extends mdr compliance timeline to 2027 2028 jpg - European Parliament Extends MDR Transition To 2027 2028 OBN Blog European Parliament Extends Mdr Compliance Timeline To 2027 2028 https www arenasolutions com wp content uploads what is MDR 2017 745 Compliance png - MDR 2017 745 Compliance Definition Arena What Is MDR 2017 745 Compliance
https connectorsupplier com wp content uploads EU MDR transition timeline jpg - EU MDR Update To Medical Device Regulations In Europe EU MDR Transition Timeline http www ce euro com uploadfiles pictures news 20221207164111 3166 jpg - MDR EU 2017 745 CE 20221207164111 3166 https medtechintelligence com wp content uploads 2018 01 EUMDR TransitionTimeline jpg - mdr eu medical device directive regulations classification revamp transition events timelines article New EU MDR Regulations And Revamp Of The Medical Device Directive EUMDR TransitionTimeline
https www qualitiso com wp content uploads 2021 09 Reglement 2017 745 demarche CE EN png - Regulation EU 2017 745 Guidance For Medical Devices Manufacturers Reglement 2017 745 Demarche CE.EN https gxp training com wp content uploads 2023 06 MDR 2017 png - Medical Device Regulation MDR 2017 745 Course And Certificate MDR 2017
https cdn01 alison static net courses 2218 alison courseware intro 2218 jpg - mdr eu regulations device medical alison essentials european EU Medical Device Regulations 2017 745 Free Online Course Alison Alison Courseware Intro 2218