Last update images today Eu Mdr Regulation Pdf
























https www fdanews com ext resources Book Covers NEW Complying with the EU MDR 500 jpg - Complying With The EU MDR New Guidances Standards And Procedures Complying With The EU MDR 500 https ds982gbwnvyso cloudfront net Pictures 1024x536 1 7 7 177 mdrfighure2 881217 jpg - Practical Insights Into The Recent EU MDR Framework Focus 177 Mdrfighure2 881217
https connectorsupplier com wp content uploads EU MDR transition timeline jpg - Medical Device Regulation MDR Will Apply From May 26 2021 40 OFF EU MDR Transition Timeline https cemarking net wp content uploads 2022 04 Changes new MDR jpg - EU MDR 2017 745 What Changed Changes New MDR https www elexes com wp content uploads 2023 07 EU MDR transition period finalized 1024x576 png - Extension To EU MDR Transition Period Finalized Elexes EU MDR Transition Period Finalized 1024x576
https www cqfluency com wp content uploads 2021 08 EU MDR Prepatation May 2021 are you prepared for language compliance png - Staying Ahead Ensuring Language Compliance As MDR S Date Of EU MDR Prepatation May 2021 Are You Prepared For Language Compliance https ds982gbwnvyso cloudfront net Pictures 1024x536 1 7 6 176 mdrfigure1 21670 jpg - Practical Insights Into The Recent EU MDR Framework Focus 176 Mdrfigure1 21670
https blog scconline gen in wp content uploads 2021 06 MDR Requirements1 jpg - EU Medical Devices Regulation MDR Comes Into Force SCC Times MDR Requirements1 https www elexes com wp content uploads 2023 07 EU MDR transition period finalized 1024x576 png - Extension To EU MDR Transition Period Finalized Elexes EU MDR Transition Period Finalized 1024x576
https www elexes com wp content uploads 2023 07 EU MDR transition period finalized 1024x576 png - Extension To EU MDR Transition Period Finalized Elexes EU MDR Transition Period Finalized 1024x576 https medicaldevicehq com wp content uploads 2022 12 eu mdr plan featured image jpg - MDR Article 22 Medical Device HQ Eu Mdr Plan Featured Image
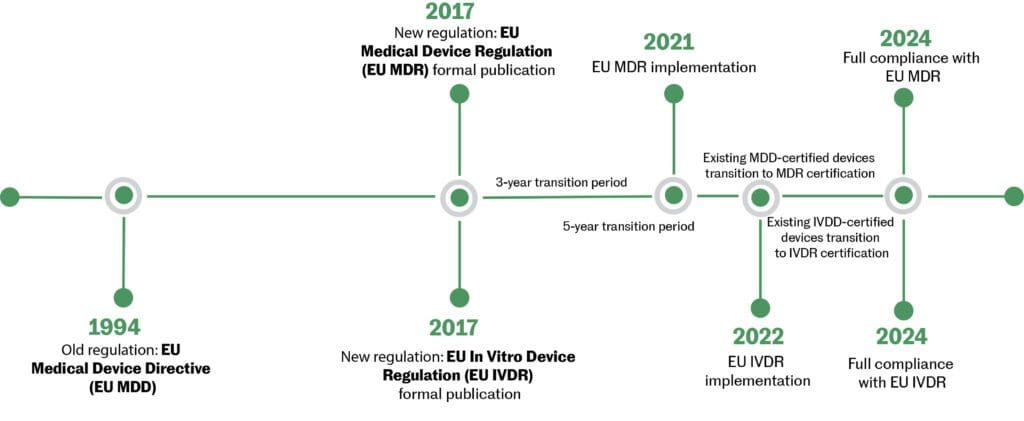
https learn marsdd com wp content uploads 2022 02 transition timeline MDD MDR 20220204 1024x421 jpg - Medical Device Regulations Classification Submissions Canada US EU Transition Timeline MDD MDR 20220204 1024x421 https www fdanews com ext resources Book Covers NEW Complying with the EU MDR 500 jpg - Complying With The EU MDR New Guidances Standards And Procedures Complying With The EU MDR 500 https cemarking net wp content uploads 2022 04 Changes new MDR jpg - EU MDR 2017 745 What Changed Changes New MDR
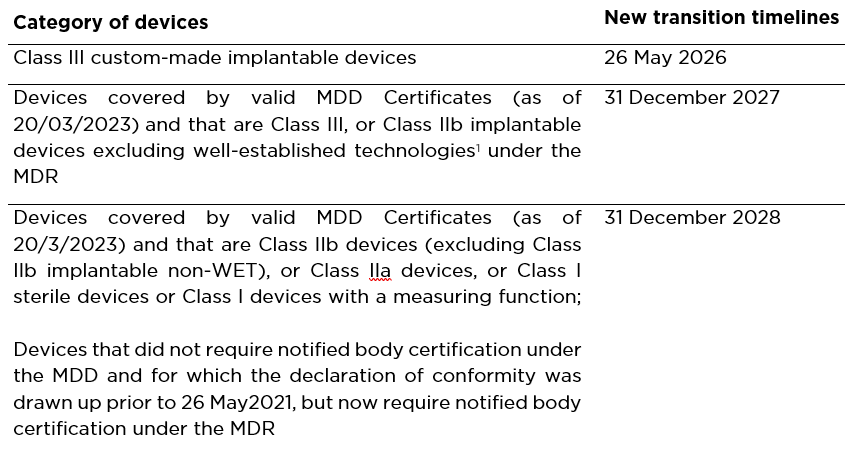
https www dqsglobal com var site storage images 2 6 0 9 2609062 1 ger DE 8bf67b8aa90c DQS MED Webseite Verlaengerung MDR jpg - MDR 2023 Extension Details Deadlines DQS USA 8bf67b8aa90c DQS MED Webseite Verlaengerung MDR http www orielstat com blog wp content uploads 2019 02 EUMDR NewTimeline2 e1591716276456 png - mdr timelines deadlines implementation regulation answered EU MDR Transition Timelines And Deadlines For 2017 745 EUMDR NewTimeline2 E1591716276456
https www elexes com wp content uploads 2023 07 EU MDR transition period finalized 1024x576 png - Extension To EU MDR Transition Period Finalized Elexes EU MDR Transition Period Finalized 1024x576 https www tuvsud com media global images resource centre infographics resource centre tuv sud mdr infographic jpg - Infographic The New Medical Device Regulation T V S D In India Tuv Sud Mdr Infographic
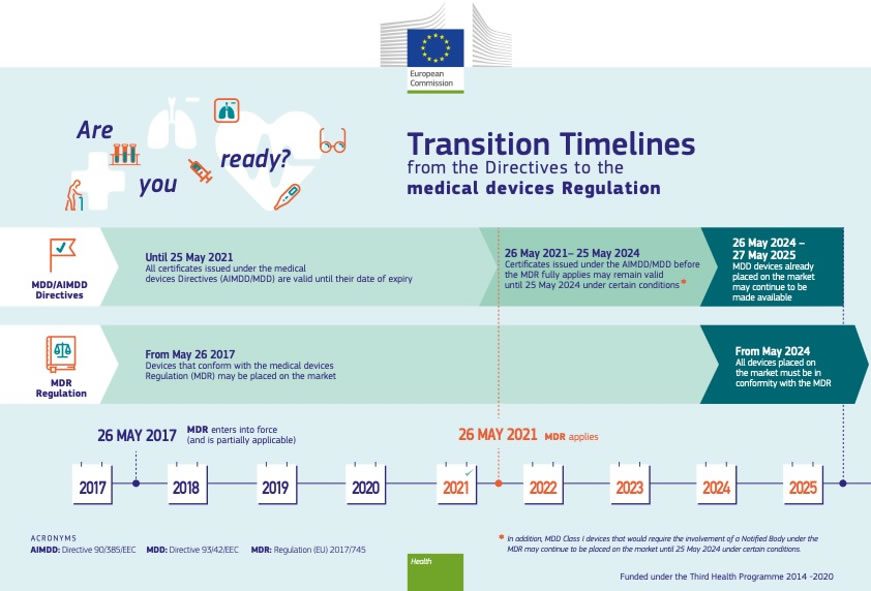
https 26728782 fs1 hubspotusercontent eu1 net hubfs 26728782 EU MDR ARE YOU READY FOR THE EU MDR jpg - MDR Europe What You Need To Know EU MDR ARE YOU READY FOR THE EU MDR https www tuvsud com media global images resource centre infographics resource centre tuv sud mdr infographic jpg - Infographic The New Medical Device Regulation T V S D In India Tuv Sud Mdr Infographic
https learn marsdd com wp content uploads 2022 02 transition timeline MDD MDR 20220204 1024x421 jpg - Medical Device Regulations Classification Submissions Canada US EU Transition Timeline MDD MDR 20220204 1024x421 https www elexes com wp content uploads 2023 07 EU MDR transition period finalized 1024x576 png - Extension To EU MDR Transition Period Finalized Elexes EU MDR Transition Period Finalized 1024x576 https www greenlight guru hs fs hubfs free download CTA cover eu mdr implementation guide png - EU MDR Implementation Guide Free Download Free Download CTA Cover Eu Mdr Implementation Guide
https www citemedical com wp content uploads 2022 02 photo 1619418602850 35ad20aa1700 jpg - EU MDR Checklist Download Get Actionable Technical Documentation Photo 1619418602850 35ad20aa1700 http www greensofttech com wp content uploads 2018 08 EU MDR featured image png - Compliance With New EU MDR In 2020 GreenSoft Technology EU MDR Featured Image