Last update images today Eu Mdr Requirements























https static wixstatic com media e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg v1 fill w 940 h 612 al c q 85 e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg - Translation And The EU MDR Requirements In 2024 E09e47 Edae1e10a72a42d791ec364bf1dac5d0~mv2 https operonstrategist com wp content uploads 2022 11 Traceability requirements for Medical Devices in EU MDR 01 1024x535 jpg - Traceability Requirements For Medical Devices In EU MDR Operon Strategist Traceability Requirements For Medical Devices In EU MDR 01 1024x535
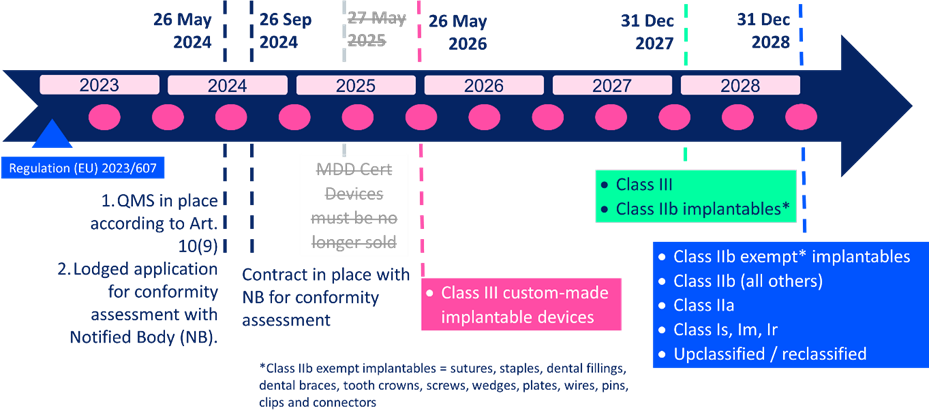
https casusconsulting com wp content uploads 2023 04 MDR Transition Guide scaled jpg - 2023 EU MDR Extension Guide Timeline FAQs Casus Consulting MDR Transition Guide Scaled https www tuvsud com media global images resource centre infographics resource centre tuv sud mdr infographic jpg - Infographic The New Medical Device Regulation T V S D In India Tuv Sud Mdr Infographic https operonstrategist com wp content uploads 2022 12 EU MDR extension UPDATE 01 1024x535 jpg - EU Proposes Extensions Of MDR Transition Period Lets Understand EU MDR Extension UPDATE 01 1024x535
https www 1med ch wp content uploads 2022 03 IMAGE 1TWEBINAR M2020 001 30MARCH jpg - TOP 5 Most Urgent Tasks For MDR Compliance Before 2024 1med IMAGE 1TWEBINAR M2020 001 30MARCH https images bannerbear com direct y0aJ23zRDdqMxX4OGl requests 000 031 622 447 NWlVkgmbMQE7rg1qzZyAqEwDo 3307be92ea9f1620e0f59d3a26d7a77eaf319ff8 png - European Commission Announces Intention To Introduce Three Year 3307be92ea9f1620e0f59d3a26d7a77eaf319ff8
https neuronewsinternational com wp content uploads sites 3 2023 03 EU cover 500x403 jpg - EU Ministers Approve Changes To MDR Transition Timetable NeuroNews EU Cover 500x403
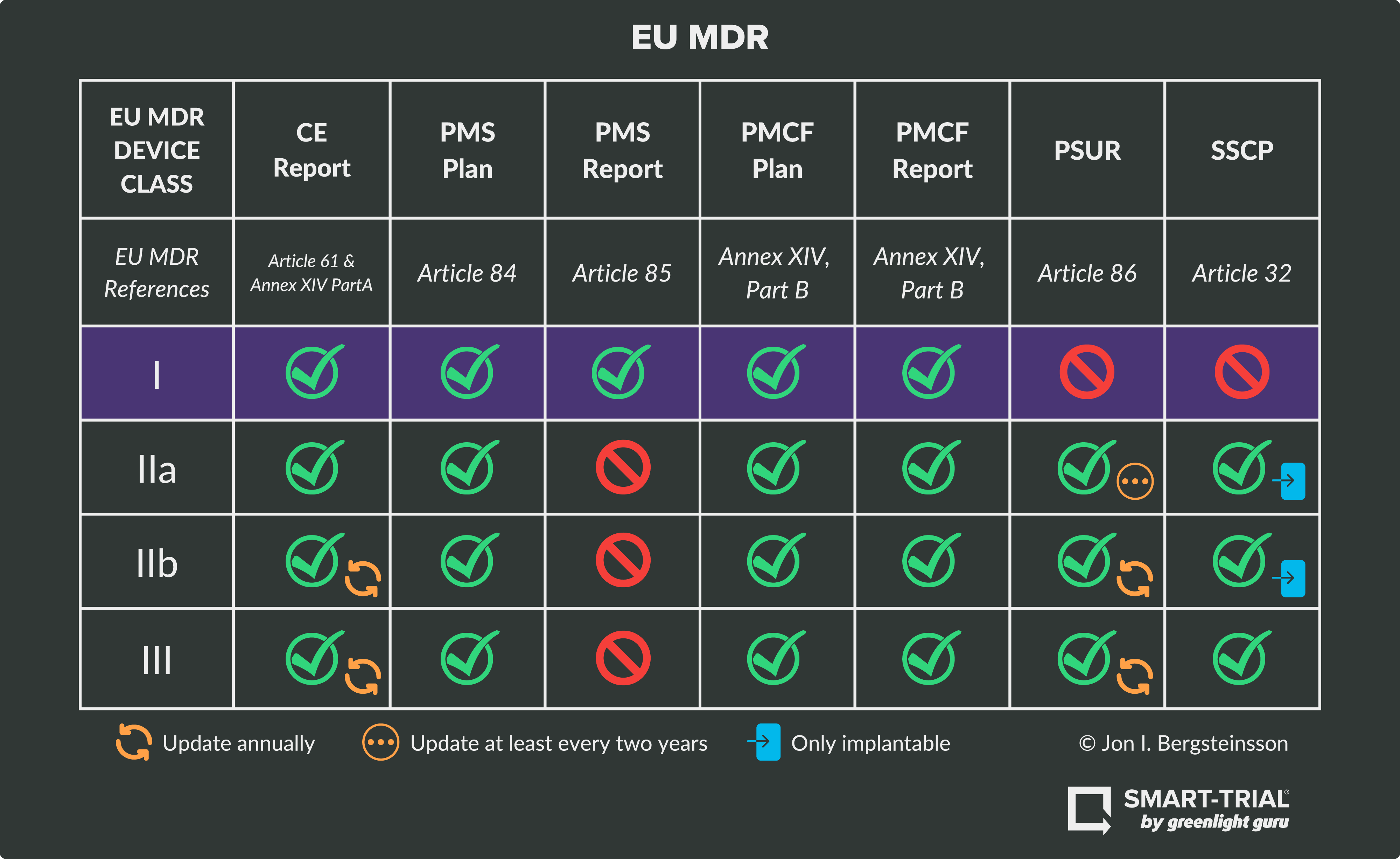
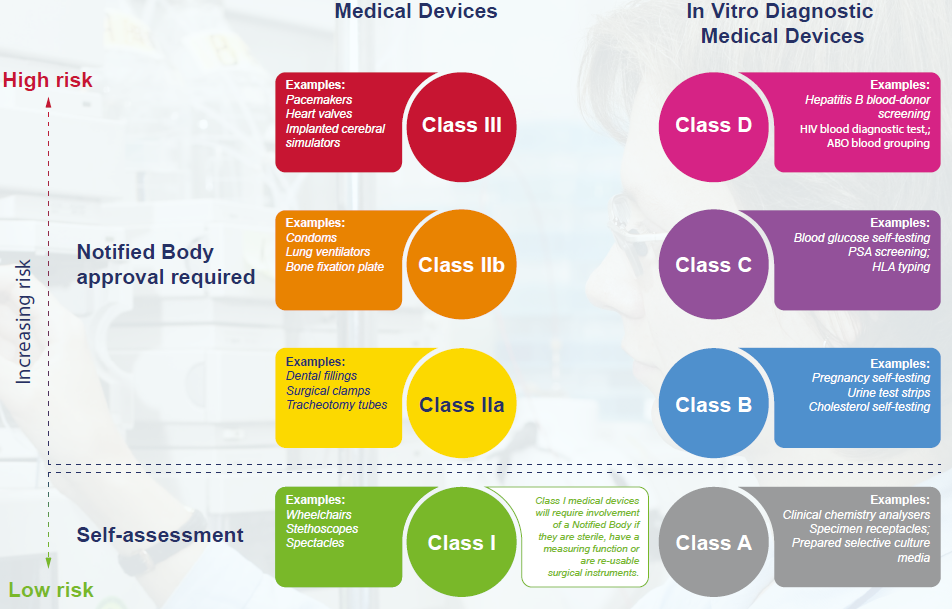
https blog greenlight guru hubfs EU MDR Class Charts 01 png - Ultimate Guide To Device Class Requirements Under EU MDR EU MDR Class Charts 01 https operonstrategist com wp content uploads 2022 11 Traceability requirements for Medical Devices in EU MDR 01 1024x535 jpg - Traceability Requirements For Medical Devices In EU MDR Operon Strategist Traceability Requirements For Medical Devices In EU MDR 01 1024x535
https www ophthalmologybreakingnews com files images blog european parliament extends mdr compliance timeline to 2027 2028 jpg - European Parliament Extends MDR Transition To 2027 2028 OBN Blog European Parliament Extends Mdr Compliance Timeline To 2027 2028 https blog greenlight guru hubfs EU MDR Class Charts 01 png - Ultimate Guide To Device Class Requirements Under EU MDR EU MDR Class Charts 01
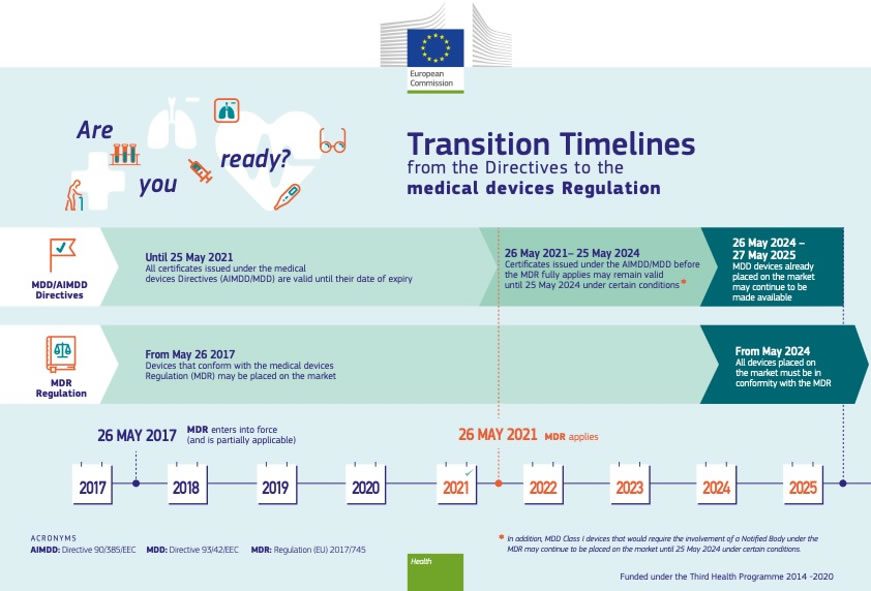
https connectorsupplier com wp content uploads EU MDR transition timeline jpg - Medical Device Regulation MDR Will Apply From May 26 2021 40 OFF EU MDR Transition Timeline https images bannerbear com direct y0aJ23zRDdqMxX4OGl requests 000 031 622 447 NWlVkgmbMQE7rg1qzZyAqEwDo 3307be92ea9f1620e0f59d3a26d7a77eaf319ff8 png - European Commission Announces Intention To Introduce Three Year 3307be92ea9f1620e0f59d3a26d7a77eaf319ff8 https emdr24 com wp content uploads 2023 09 25thanniversay emdr 2 480x480 png - EMDR 2024 25thanniversay Emdr 2 480x480
https www biosliceblog com wp content uploads sites 429 2017 09 Medical devices pic2 png - medical devices eu mhra regulations classification device guide system interactive MHRA S Guide To The New EU Medical Devices Regulations BioSlice Blog Medical Devices Pic2 https www 1med ch wp content uploads 2022 03 IMAGE 1TWEBINAR M2020 001 30MARCH jpg - TOP 5 Most Urgent Tasks For MDR Compliance Before 2024 1med IMAGE 1TWEBINAR M2020 001 30MARCH
https acquiscompliancewebprod blob core windows net acquiscomplianceweb assets EU MDR and IVDR 00197df807 jpg - EU MDR Compliance Key Requirements For Medical Devices EU MDR And IVDR 00197df807
https blog greenlight guru hubfs EU MDR Class Charts 01 png - Ultimate Guide To Device Class Requirements Under EU MDR EU MDR Class Charts 01 https www orielstat com blog wp content uploads 2021 03 eumdr graphic e1614962121251 jpg - Understanding EU IVD Performance Evaluation Plan And Report Eumdr Graphic E1614962121251
https cemarking net wp content uploads 2022 04 Changes new MDR jpg - EU MDR 2017 745 What Changed Changes New MDR http www orielstat com blog wp content uploads 2019 02 EUMDR NewTimeline2 e1591716276456 png - mdr timelines deadlines implementation regulation answered EU MDR Transition Timelines And Deadlines For 2017 745 EUMDR NewTimeline2 E1591716276456
https static wixstatic com media e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg v1 fill w 940 h 612 al c q 85 e09e47 edae1e10a72a42d791ec364bf1dac5d0 mv2 jpg - Translation And The EU MDR Requirements In 2024 E09e47 Edae1e10a72a42d791ec364bf1dac5d0~mv2 https www eucope org wp content uploads 2023 01 news posts 1024x576 png - MDR IVDR Implementation EC S Proposal On 6 January 2023 News Posts 1024x576 https operonstrategist com wp content uploads 2022 12 EU MDR extension UPDATE 01 1024x535 jpg - EU Proposes Extensions Of MDR Transition Period Lets Understand EU MDR Extension UPDATE 01 1024x535
https www biosliceblog com wp content uploads sites 429 2017 09 Medical devices pic2 png - medical devices eu mhra regulations classification device guide system interactive MHRA S Guide To The New EU Medical Devices Regulations BioSlice Blog Medical Devices Pic2 https i ytimg com vi HKkrR5Nzkl0 maxresdefault jpg - Is The EU MDR Now Extended What If Your Certificates Already Expired Maxresdefault
https connectorsupplier com wp content uploads EU MDR transition timeline jpg - Medical Device Regulation MDR Will Apply From May 26 2021 40 OFF EU MDR Transition Timeline