Last update images today Eu Mdr Significant Change
















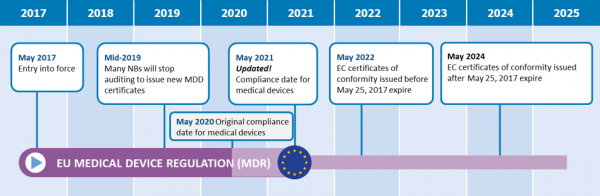






https www s3connectedhealth com hs fs hubfs mdr eu 2021 jpg - mdr device medical classification eu rule 2021 MDR Rule 11 What The Change Means For Medical Device Companies Mdr Eu 2021 https inglasia com wp content uploads 2022 03 EU MDR timeline 1 768x799 png - MDR Transition A Compliant Approach To Current MDR Inglasia EU MDR Timeline 1 768x799
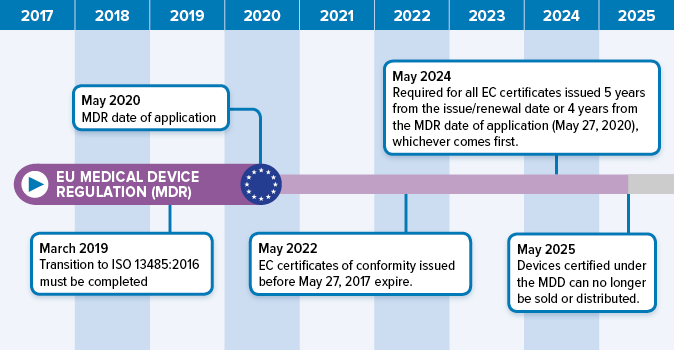
https www mylanguageconnection com wp content uploads 2020 01 EU MDR Timeline png - mdr regulation translation affect process The EU Medical Device Regulation EU MDR My Language Connection EU MDR Timeline https www jamasoftware com media 2021 12 2021 12 28 new eumdr regulations 1024x512 1 jpg - Takeaways What Changes To The EU MDR Mean For You Jama Software 2021 12 28 New Eumdr Regulations 1024x512 1 https mastermindtranslations co uk wp content uploads 2022 04 MDR Language Requirements Feb 2024 v1 0 768x543 png - EU MDR Language Requirements Table For Medical Devices 2024 MDR Language Requirements Feb 2024 V1.0 768x543
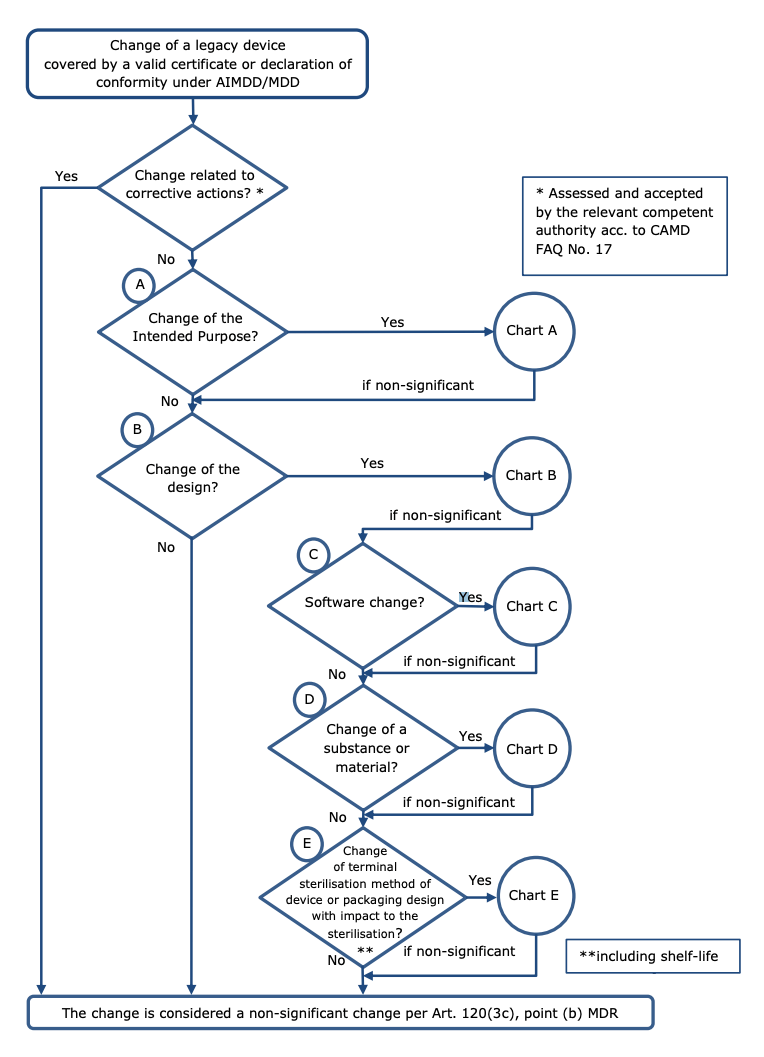
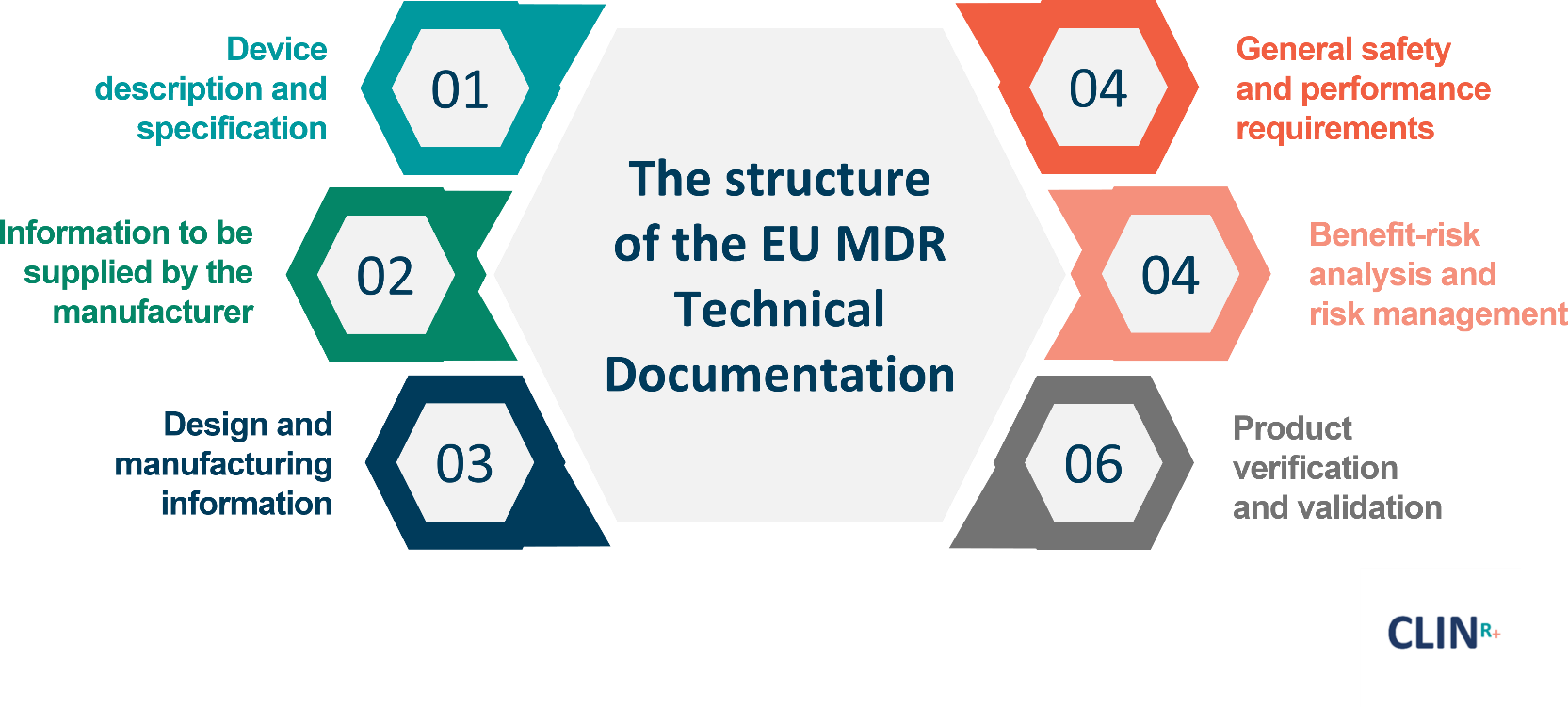
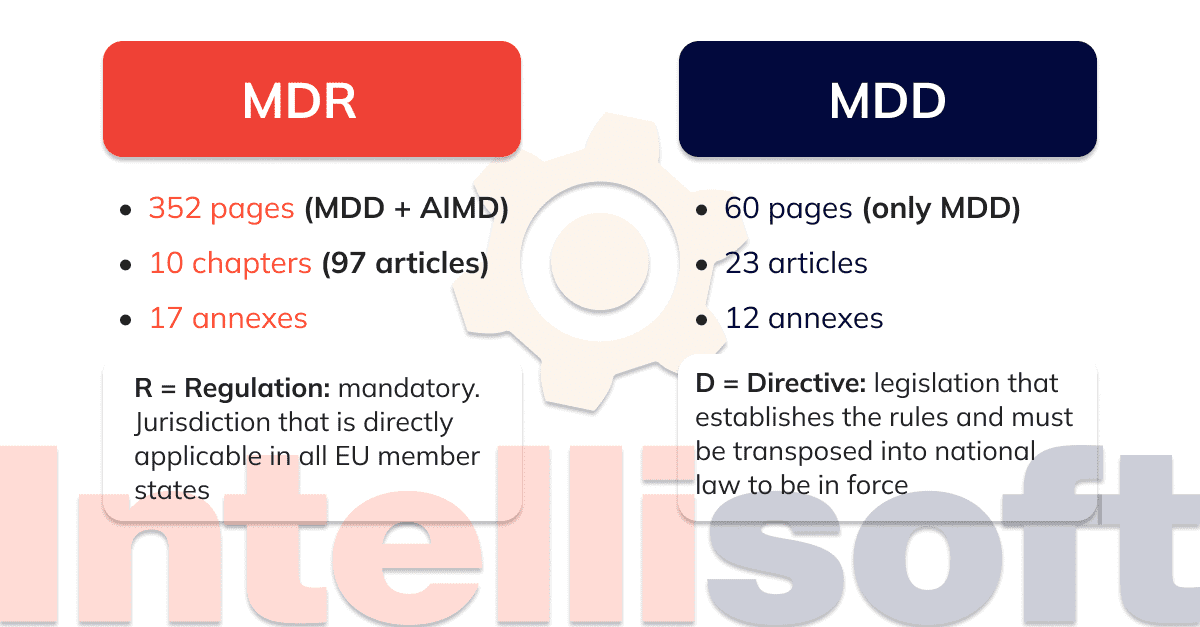
https i0 wp com mdlaw eu wp content uploads 2023 05 Chart C png - MDCG Guidance On Significant Changes MDR Provisions Chart C https intellisoft io wp content uploads 2022 06 2 MDR with MDD png - EU MDR MDD Key Differences Infographic 46 OFF 2 MDR With MDD
https www rqmplus com hubfs ChangeCompressed jpg - mdr New EU MDR Guidance On Significant Changes ChangeCompressed
https www s3connectedhealth com hs fs hubfs mdr eu 2021 jpg - mdr device medical classification eu rule 2021 MDR Rule 11 What The Change Means For Medical Device Companies Mdr Eu 2021 https image slidesharecdn com wolf eu mdr omtec 2018 180620191821 85 eu mdr preparation seize the market opportunity and avoid the bottleneck 3 320 jpg - EU MDR Preparation Seize The Market Opportunity And Avoid The Eu Mdr Preparation Seize The Market Opportunity And Avoid The Bottleneck 3 320
https www rqmplus com hubfs ChangeCompressed jpg - mdr New EU MDR Guidance On Significant Changes ChangeCompressed https mastermindtranslations co uk wp content uploads 2022 04 MDR Language Requirements Feb 2024 v1 0 768x543 png - EU MDR Language Requirements Table For Medical Devices 2024 MDR Language Requirements Feb 2024 V1.0 768x543
https www scilife io hs fs hubfs SL MDR 4 jpg - mdr lays regulation EU MDR Key Changes And Important Steps Scilife SL MDR 4 https i0 wp com mdlaw eu wp content uploads 2023 05 Chart D png - MDCG Guidance On Significant Changes MDR Provisions Chart D https www scilife io hs fs hubfs MDR v01 1 jpg - EU MDR Key Changes And Important Steps Scilife MDR V01 1
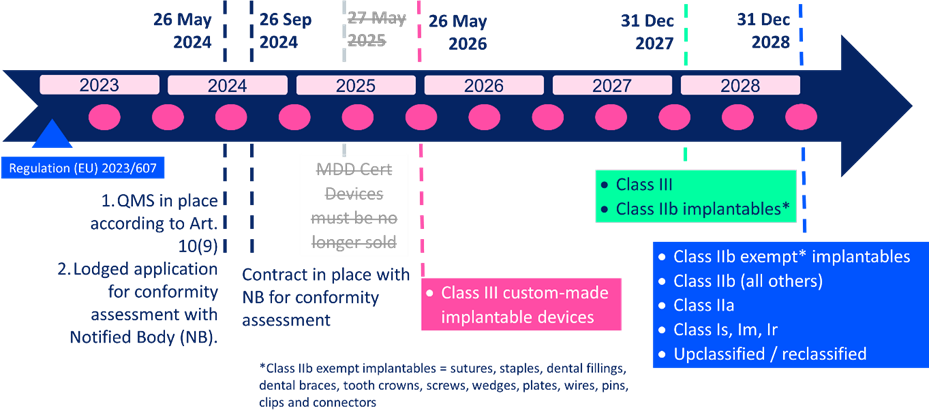
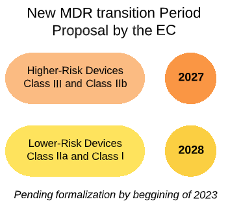
https criticalcatalyst com wp content uploads 2022 12 MDR postponed EN png - EU MDR Proposal For Extension Of Transition Period CRITICAL CATALYST MDR Postponed EN https casusconsulting com wp content uploads 2023 11 Significant Changes MDR IVDR legacy devices scaled jpg - 2024 MDR IVDR Legacy Device Significant Change Tool Casus Consulting Significant Changes MDR IVDR Legacy Devices Scaled
https www s3connectedhealth com hs fs hubfs mdr eu 2021 jpg - mdr device medical classification eu rule 2021 MDR Rule 11 What The Change Means For Medical Device Companies Mdr Eu 2021
https cdn slidesharecdn com ss thumbnails significantchangesmdrv02 200306072549 thumbnail jpg - MDR Significant Changes In The Design And Intended Purpose PPT Significantchangesmdrv02 200306072549 Thumbnail https casusconsulting com wp content uploads 2023 11 Significant Changes MDR IVDR legacy devices scaled jpg - 2024 MDR IVDR Legacy Device Significant Change Tool Casus Consulting Significant Changes MDR IVDR Legacy Devices Scaled
https mastermindtranslations co uk wp content uploads 2022 04 MDR Language Requirements Feb 2024 v1 0 768x543 png - EU MDR Language Requirements Table For Medical Devices 2024 MDR Language Requirements Feb 2024 V1.0 768x543 https www s3connectedhealth com hs fs hubfs mdr eu 2021 jpg - mdr device medical classification eu rule 2021 MDR Rule 11 What The Change Means For Medical Device Companies Mdr Eu 2021
https www scilife io hs fs hubfs SL MDR 2 jpg - mdr EU MDR Key Changes And Important Steps Scilife SL MDR 2 https i0 wp com mdlaw eu wp content uploads 2023 05 Chart B png - MDCG Guidance On Significant Changes MDR Provisions Chart B https www scilife io hs fs hubfs SL MDR 4 jpg - mdr lays regulation EU MDR Key Changes And Important Steps Scilife SL MDR 4
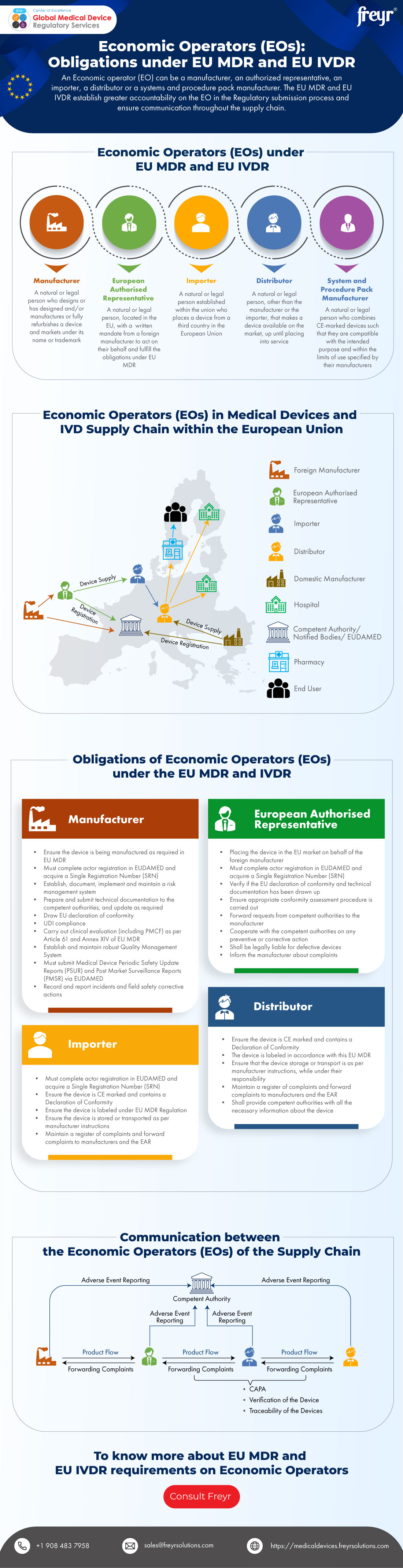
https i0 wp com mdlaw eu wp content uploads 2023 05 Chart C png - MDCG Guidance On Significant Changes MDR Provisions Chart C https www freyrsolutions com sites default files infographics economic operators eos obligations under eu mdr and eu ivdr jpg - Economic Operators EOs Obligations Under EU MDR And EU IVDR Freyr Economic Operators Eos Obligations Under Eu Mdr And Eu Ivdr
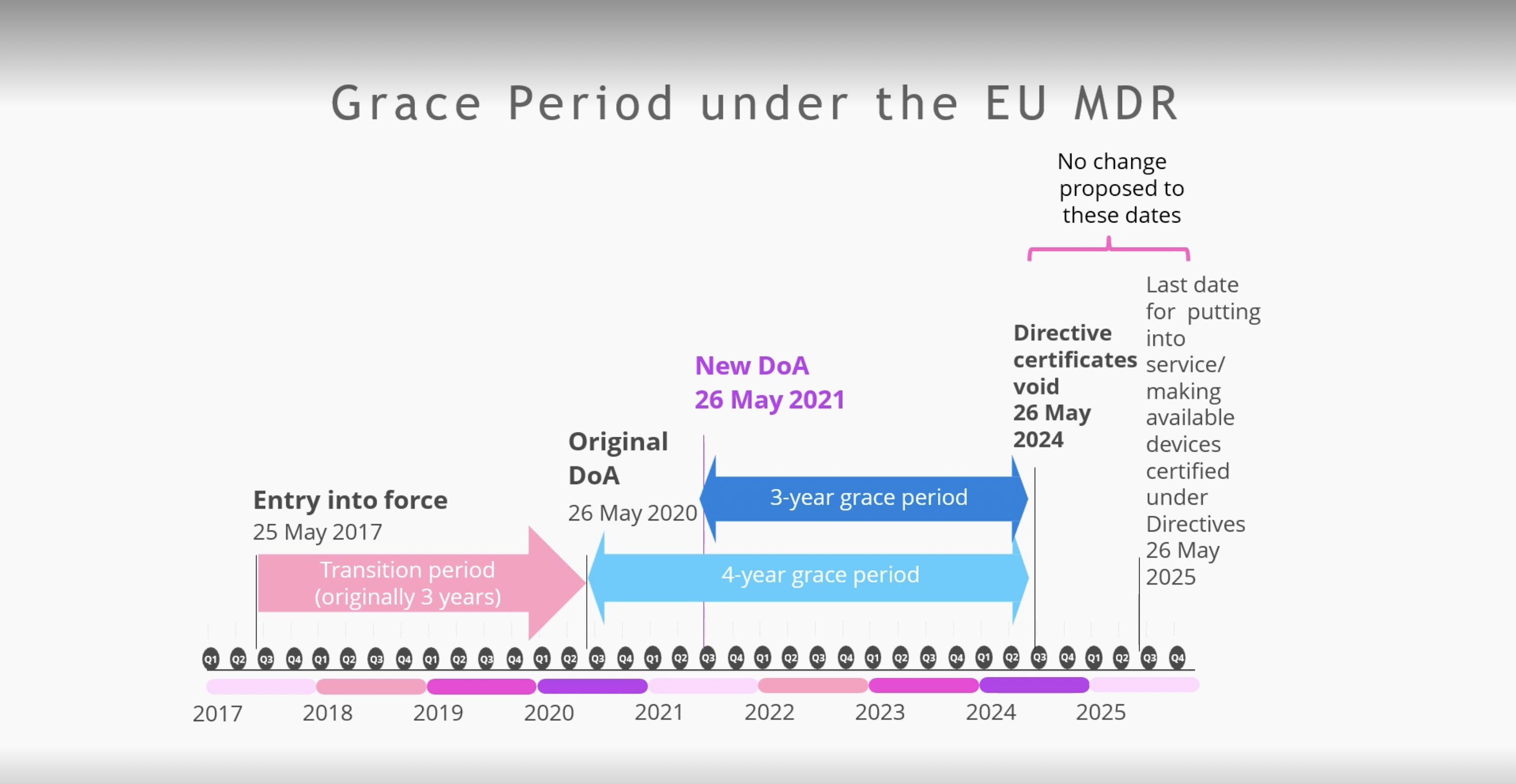
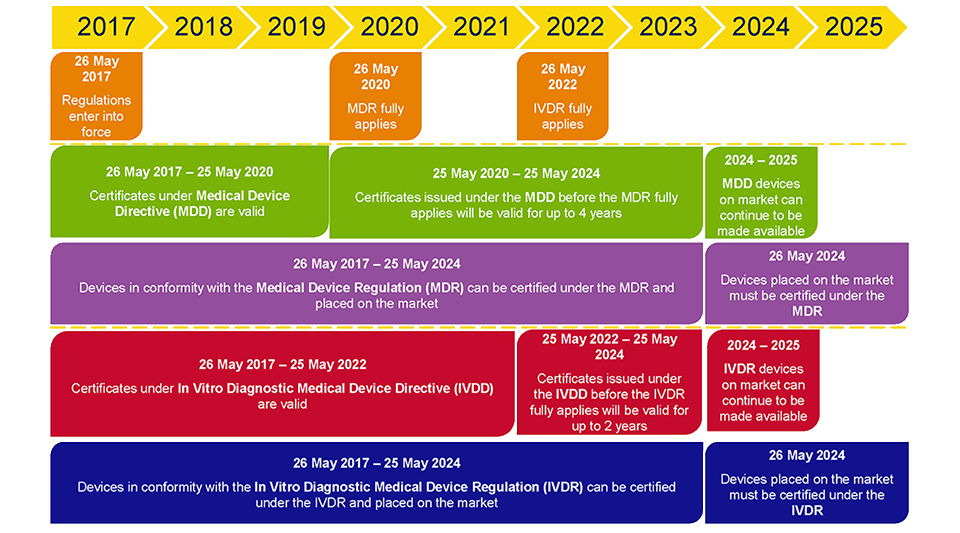
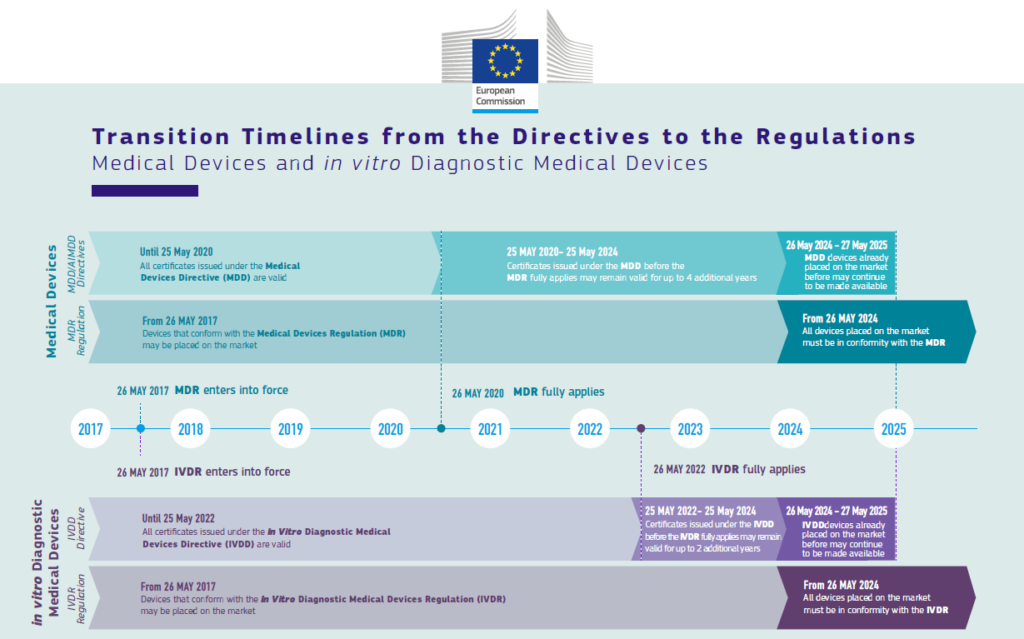
https www orielstat com blog wp content uploads 2018 03 EU MDR Transition v2 png - mdr eu transition timeline medical device regulation mdd timelines changes directive difference ce overview marking compliance EU MDR Transition Timelines And Deadlines For 2017 745 EU MDR Transition V2