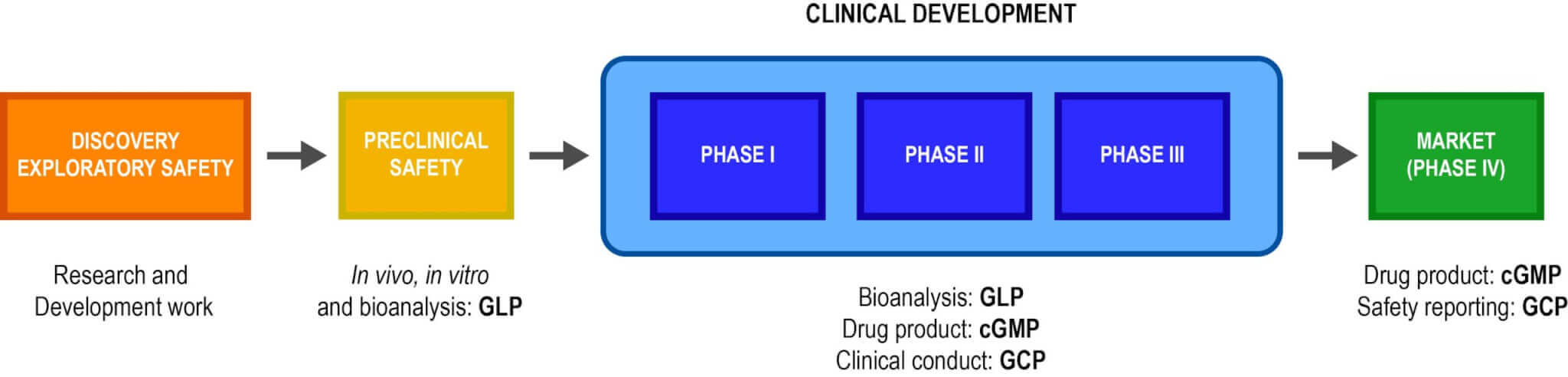
Last update images today Glp Regulations 21 Cfr Part 58





























https www coursehero com thumb e9 9e e99e9511472733de88c7bb7aaf7c240c1a4f1e87 180 jpg - Understanding GLP Regulations Safety Scope Definitions And Course E99e9511472733de88c7bb7aaf7c240c1a4f1e87 180 https www labelmaster com product image DOT0162 jpg - Cfr Books 2024 Ginni Justine DOT0162
https blogger googleusercontent com img b R29vZ2xl AVvXsEj9XDFEPQ d81B6isbKik1bHvTT GEZdfvvPrTNcG wu 6uPHpgbkV4qTpMW2LfHp 7iCxZB O1wo48w3hwczhPTX3dtm kMzwr Q0jIUBEBD3W9up9M8fyBLj3V30xfAJDDsmNpEosj43aMwSA5XfbrEUj9KPIm3p2WTFj9IqwC BJhl3 vO1BYo3YXA s441 10Part210 jpg - 21 CFR Part 210 And 211 10Part210 https image slidesharecdn com dataintegrity 170128154223 85 21 cfr part 58 data integrity 2 320 jpg - 21 CFR Part 58 Data Integrity PPT 21 Cfr Part 58 Data Integrity 2 320 https www gmppublications com borders GXPNews copy2 jpg - cfr part glp 21 CFR Part 58 GLP Good Laboratory Practice At GMP Publications Store GXPNews Copy2
https is1 ssl mzstatic com image thumb Purple115 v4 dc 02 c8 dc02c84e 0e89 0bc2 145d eb848a28a9f3 AppIcon 0 0 1x U007emarketing 0 0 0 5 0 0 sRGB 0 0 0 GLES2 U002c0 512MB 85 220 0 0 png 1200x630wa png - App Store 21 CFR Part 58 1200x630wa https pic1 zhimg com v2 39ef4e35981fdbd2bd0ea19a7b863338 r jpg - FDA V2 39ef4e35981fdbd2bd0ea19a7b863338 R
http image3 slideserve com 6792971 glp general requirements n jpg - cfr part good glp laboratory practice requirements general slideserve PPT Good Laboratory Practice CFR 21 Part 58 PowerPoint Presentation Glp General Requirements N
https images hgmsites net hug 2024 mercedes benz glb class facelift spy shots photo credits baldauf sb medien 100850289 h jpg - 2024 Mercedes Benz GLB Class Spy Shots Boxy Compact Crossover Due For 2024 Mercedes Benz Glb Class Facelift Spy Shots Photo Credits Baldauf Sb Medien 100850289 H https img dokumen tips doc image 608e72c3cd4d704af4484c39 statement of glp quality assurance activities for nonclinical laboratory studies jpg - PDF STATEMENT OF GLP QUALITY ASSURANCE ACTIVITIES FOR NONCLINICAL Statement Of Glp Quality Assurance Activities For Nonclinical Laboratory Studies
https pic4 zhimg com v2 90aab596c3bd2ec8aeb2e6e8043f939f b jpg - FDA V2 90aab596c3bd2ec8aeb2e6e8043f939f B https images hgmsites net hug 2024 mercedes benz glb class facelift spy shots photo credits baldauf sb medien 100850289 h jpg - 2024 Mercedes Benz GLB Class Spy Shots Boxy Compact Crossover Due For 2024 Mercedes Benz Glb Class Facelift Spy Shots Photo Credits Baldauf Sb Medien 100850289 H
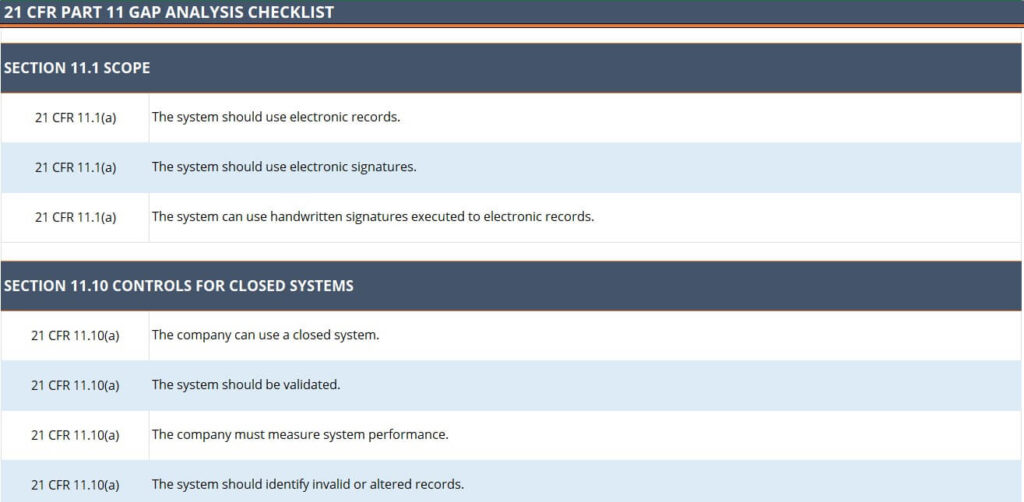
https image slidesharecdn com glp21cfrpart58 200218043415 85 glp 21 cfr part 58 2 320 jpg - GLP 21 CFR Part 58 PPT Glp 21 Cfr Part 58 2 320 https img doc xuehai net pic 28f087c5ff2ad5ca2edcb8e8 1 810 jpg 6 1080 0 0 1080 jpg - EPA Good Laboratory Practice CFR Part 160 FIFRA GLP 1 810 Jpg 6 1080 0 0 1080 https www labpeople com wp content uploads 2023 02 wl 11231 e jpg - GLP 21 CFR 11 Compliance Checklist Lab People Wl 11231 E
https joyanswer org upload 114 regulatory compliance in testing glp and gmp guide jpg - Regulatory Compliance In Testing GLP And GMP Guide JoyAnswer Org Regulatory Compliance In Testing Glp And Gmp Guide https pic1 zhimg com v2 39ef4e35981fdbd2bd0ea19a7b863338 r jpg - FDA V2 39ef4e35981fdbd2bd0ea19a7b863338 R
https file2 zhuangpeitu com fileroot2 2021 9 13 273a92f0 c015 404a be13 27f532c8f026 273a92f0 c015 404a be13 27f532c8f0263 gif - 4334565663GLP FDA 21 CFR Part 58 273a92f0 C015 404a Be13 27f532c8f0263
http qmsdoc com wp content uploads 2019 02 CFR 2 png - 21 CFR Part 801 Labeling QMS Templates CFR 2 https image5 slideserve com 9311256 chapter 1 title 21 cfr 58 subpart a general l jpg - general regulations cfr subpart PPT General Overview Of Code Of Regulations 21 Part 58 Good Chapter 1 Title 21 Cfr 58 Subpart A General L
http image3 slideserve com 6792971 glp general requirements n jpg - cfr part good glp laboratory practice requirements general slideserve PPT Good Laboratory Practice CFR 21 Part 58 PowerPoint Presentation Glp General Requirements N https cdn slidesharecdn com ss thumbnails glp21cfrpart58 200218043415 thumbnail jpg - GLP 21 CFR Part 58 PPT Glp21cfrpart58 200218043415 Thumbnail
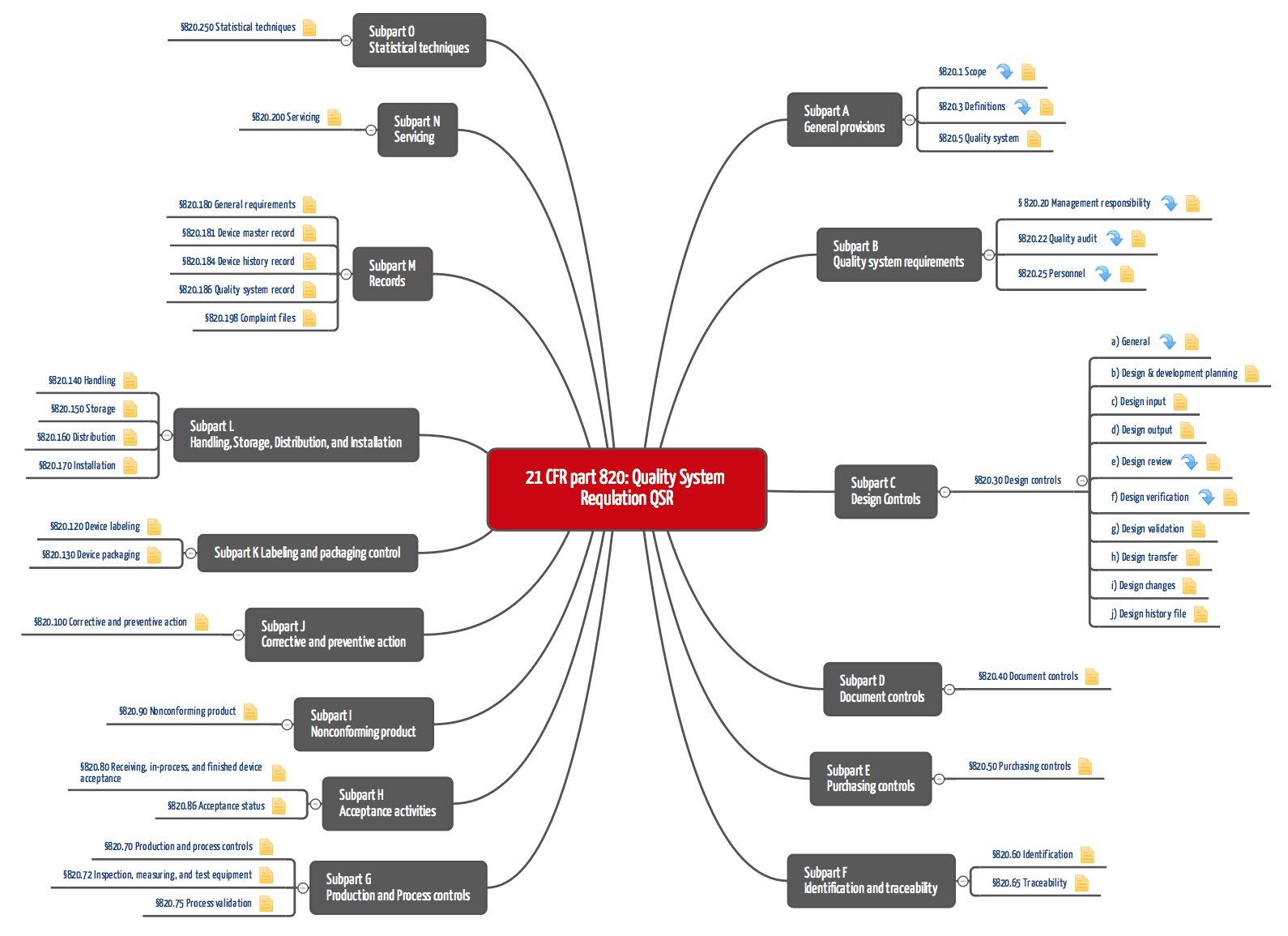
https img doc xuehai net pic 28f087c5ff2ad5ca2edcb8e8 1 810 jpg 6 1080 0 0 1080 jpg - EPA Good Laboratory Practice CFR Part 160 FIFRA GLP 1 810 Jpg 6 1080 0 0 1080 https www johner institute com fileadmin user upload 21 CFR part 820 QSR png - FDA Requirements For Quality Management Systems 21 CFR Part 820 QSR https gxp training com wp content uploads 2022 11 FDA 21 CFR 820 training 768x538 png - GMP For Medical Devices Online Training FDA 21 CFR PART 820 FDA 21 CFR 820 Training 768x538
https image slidesharecdn com dataintegrity 170128154223 85 21 cfr part 58 data integrity 27 320 jpg - 21 CFR Part 58 Data Integrity PPT 21 Cfr Part 58 Data Integrity 27 320 https bookstore gpo gov sites default files covers code of federal regulations cfr january 1 2022 31 png - Cfr Title 8 Code Of Federal Regulations 2022 U S Government Bookstore Code Of Federal Regulations Cfr January 1 2022 31
https www gmppublications com borders GXPNews copy2 jpg - cfr part glp 21 CFR Part 58 GLP Good Laboratory Practice At GMP Publications Store GXPNews Copy2