Last update images today Ivdr Regulations Qms


















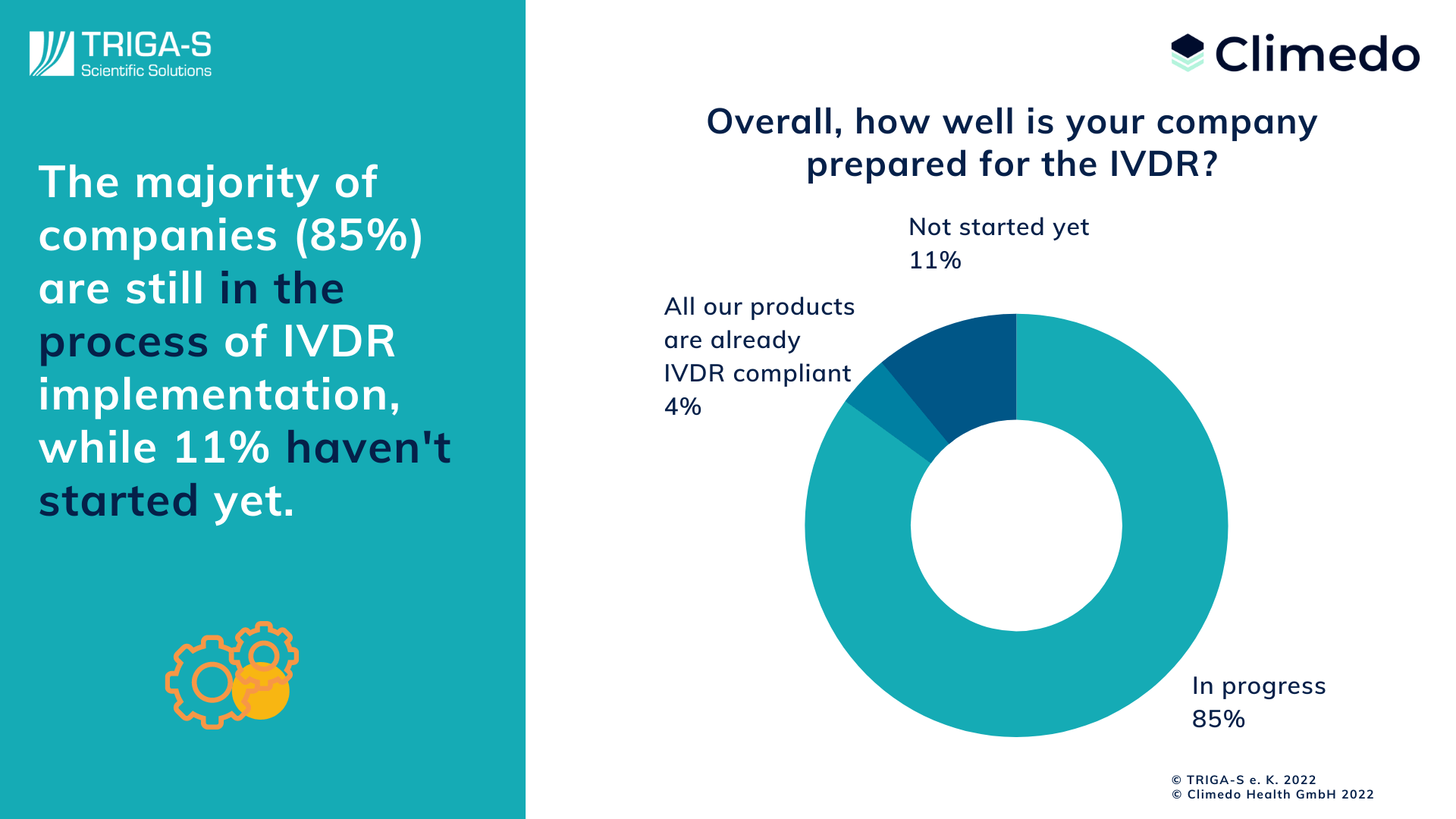







https climedo de wp content uploads 2022 04 IVDR Survey Results Preparation png - Results Of The IVDR Survey 2022 Climedo IVDR Survey Results Preparation https mdlaw eu wp content uploads 2021 09 IVDR Regulatory Strategy pdf jpg - IVDR Regulatory Strategy Implementation Guide IVDR Regulatory Strategy Pdf
https vertassets blob core windows net image 51584d7e 51584d7e d23d 4559 a5ae b5c2ac59a0bb ivdr jpg - ivdr The 6 Step Checklist For IVDR Compliance Ivdr https veranex com wp content uploads 2024 01 image3c webp - 2024 IVDR Sprint Image3c.webphttps veranex com wp content uploads 2024 01 image7 webp - 2024 IVDR Sprint Image7.webp
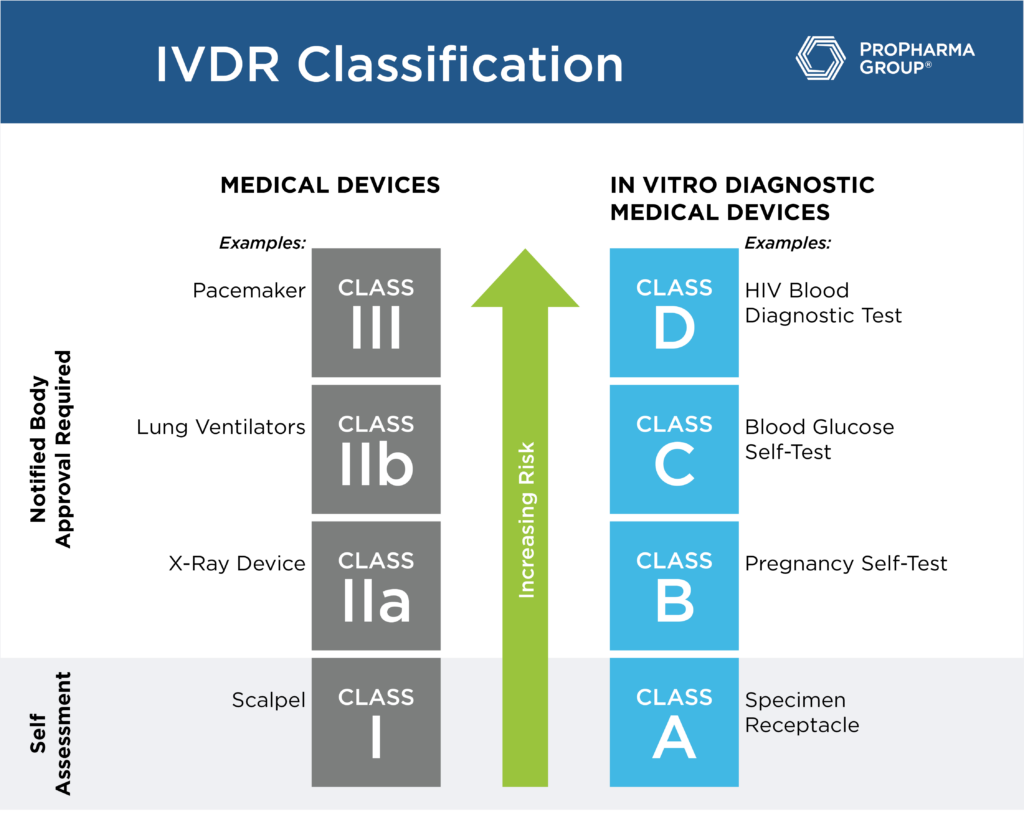
https www qservegroup com write Afbeeldingen1 IVDR design 800 Qserve IVDR remediation jpg ashx - Key Changes IVDD IVDR Gap Analysis 800 Qserve IVDR Remediation .ashxhttps www propharmagroup com hubfs Imported Blog Media IVDR Classification 1024x823 4 2 png - Roadmap For Successful IVDR Transition IVDR Classification 1024x823 4 2
https episkeymedical com wp content uploads 2021 10 pexels roberto carrafa 3908179 scaled jpg - IVDR Regulation Postponed To 2025 27 Episkey Medical Consulting Pexels Roberto Carrafa 3908179 Scaled
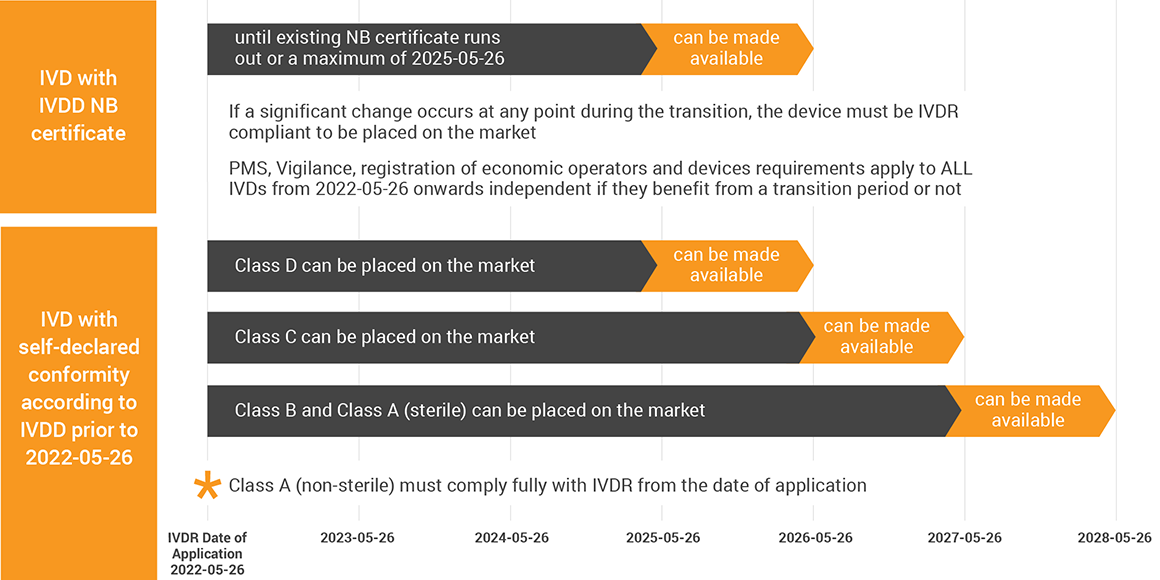
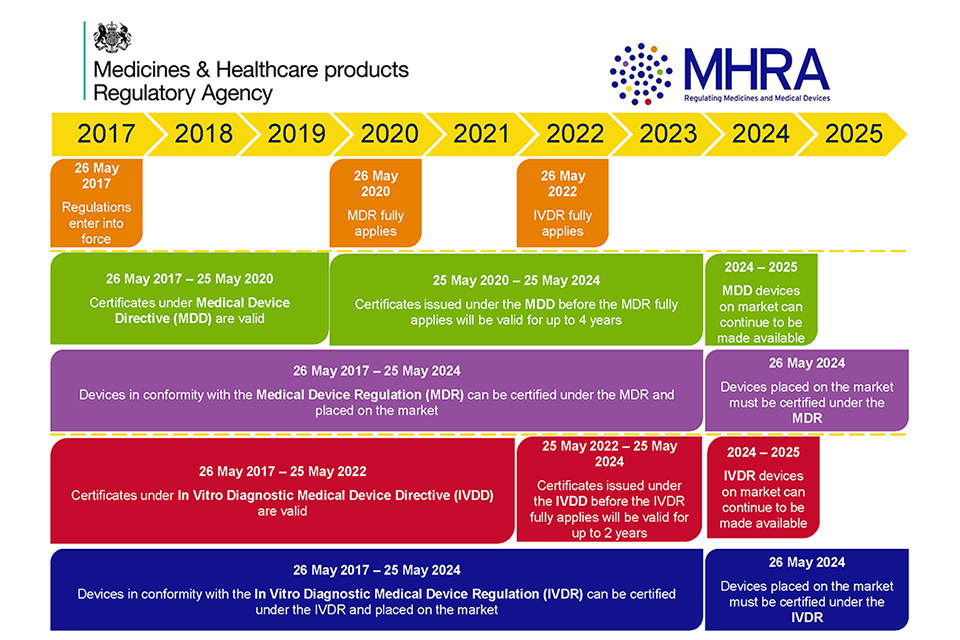
https www tuvsud com media global images industries healthcare and medical device webpages ivdr faq ivdr faq timeline of regulation 2022112 jpg - EU In Vitro Diagnostic Medical Device Regulation T V S D Ivdr Faq Timeline Of Regulation 2022112 https qtics group storage news June2022 10 steps IVDR final jpg2 jpg - IVDR Current Status News QTICS Group 10 Steps IVDR Final 2
https www propharmagroup com hubfs Imported Blog Media IVDR Classification 1024x823 4 2 png - Roadmap For Successful IVDR Transition IVDR Classification 1024x823 4 2 https climedo de wp content uploads 2022 04 IVDR Survey Results Preparation png - Results Of The IVDR Survey 2022 Climedo IVDR Survey Results Preparation
https veranex com wp content uploads 2024 01 image7 webp - 2024 IVDR Sprint Image7.webphttps images squarespace cdn com content v1 5a73786d8c56a8c8dc5ff54e e37bafe7 1bde 418f a427 18ea576b4c04 IVDR png - 5 Key Implications From The New EU In Vitro Diagnostics Regulation IVDR https www veeva com medtech wp content uploads 2022 01 IVDR Proposal Article Graphic png - How The IVDR Transition Impacts You Veeva MedTech IVDR Proposal Article Graphic
https assets publishing service gov uk government uploads system uploads image data file 66243 MHRA Certificate Validity 960x640 png - medical mdr ivdr devices eu regulations period ce certificate directives gov mhra under date transition guidance four after file issued Medical Devices EU Regulations For MDR And IVDR GOV UK MHRA Certificate Validity 960x640 https episkeymedical com wp content uploads 2021 10 pexels roberto carrafa 3908179 scaled jpg - IVDR Regulation Postponed To 2025 27 Episkey Medical Consulting Pexels Roberto Carrafa 3908179 Scaled
https www qservegroup com write Afbeeldingen1 IVDR design 800 Qserve IVDR remediation jpg ashx - Key Changes IVDD IVDR Gap Analysis 800 Qserve IVDR Remediation .ashx
https assets publishing service gov uk government uploads system uploads image data file 66243 MHRA Certificate Validity 960x640 png - medical mdr ivdr devices eu regulations period ce certificate directives gov mhra under date transition guidance four after file issued Medical Devices EU Regulations For MDR And IVDR GOV UK MHRA Certificate Validity 960x640 https veranex com wp content uploads 2024 01 image3c webp - 2024 IVDR Sprint Image3c.webp
https www propharmagroup com hubfs Imported Blog Media IVDR Classification 1024x823 4 2 png - Roadmap For Successful IVDR Transition IVDR Classification 1024x823 4 2 https climedo de wp content uploads 2022 04 IVDR Survey Results Preparation png - Results Of The IVDR Survey 2022 Climedo IVDR Survey Results Preparation
https www meditrial net wp content uploads 2022 02 ivdr jpg - MDCG Updates The IVDR Implementation And Preparation Plan EU Meditrial Ivdr https cemarking net wp content uploads 2022 05 IVDR Surveillance guidelines MDCG 2022 6 jpg - MDCG Publishes IVDR Guidelines MDCG 2022 6 IVDR Surveillance Guidelines MDCG 2022 6 https lifesciences danaher com content dam danaher blogs external blogs ivdr deadline jpg - Preparing For IVDR 2024 Deadline For Laboratories Why You Must Act Now Ivdr Deadline
https assets publishing service gov uk government uploads system uploads image data file 66243 MHRA Certificate Validity 960x640 png - medical mdr ivdr devices eu regulations period ce certificate directives gov mhra under date transition guidance four after file issued Medical Devices EU Regulations For MDR And IVDR GOV UK MHRA Certificate Validity 960x640 https vertassets blob core windows net image 51584d7e 51584d7e d23d 4559 a5ae b5c2ac59a0bb ivdr jpg - ivdr The 6 Step Checklist For IVDR Compliance Ivdr
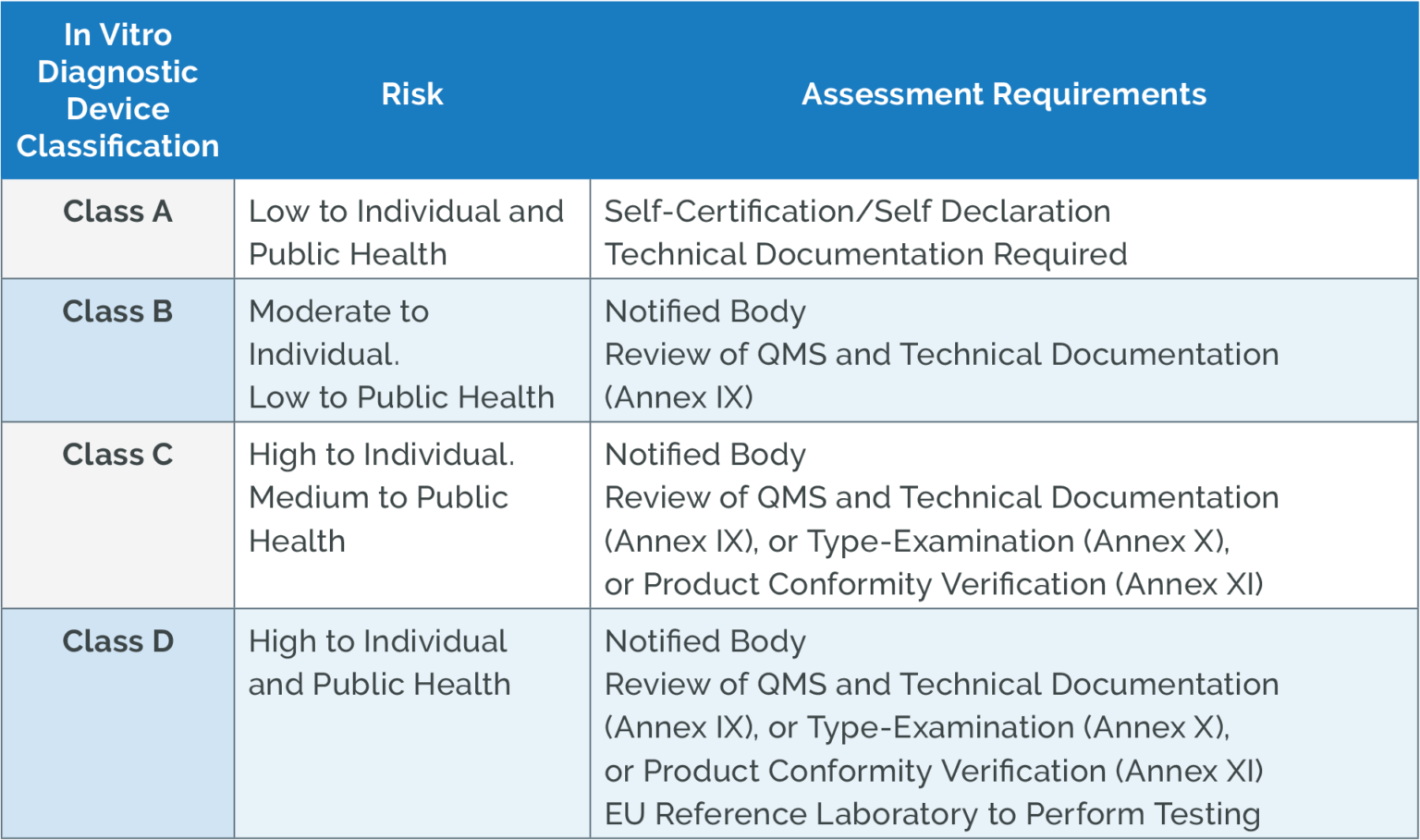
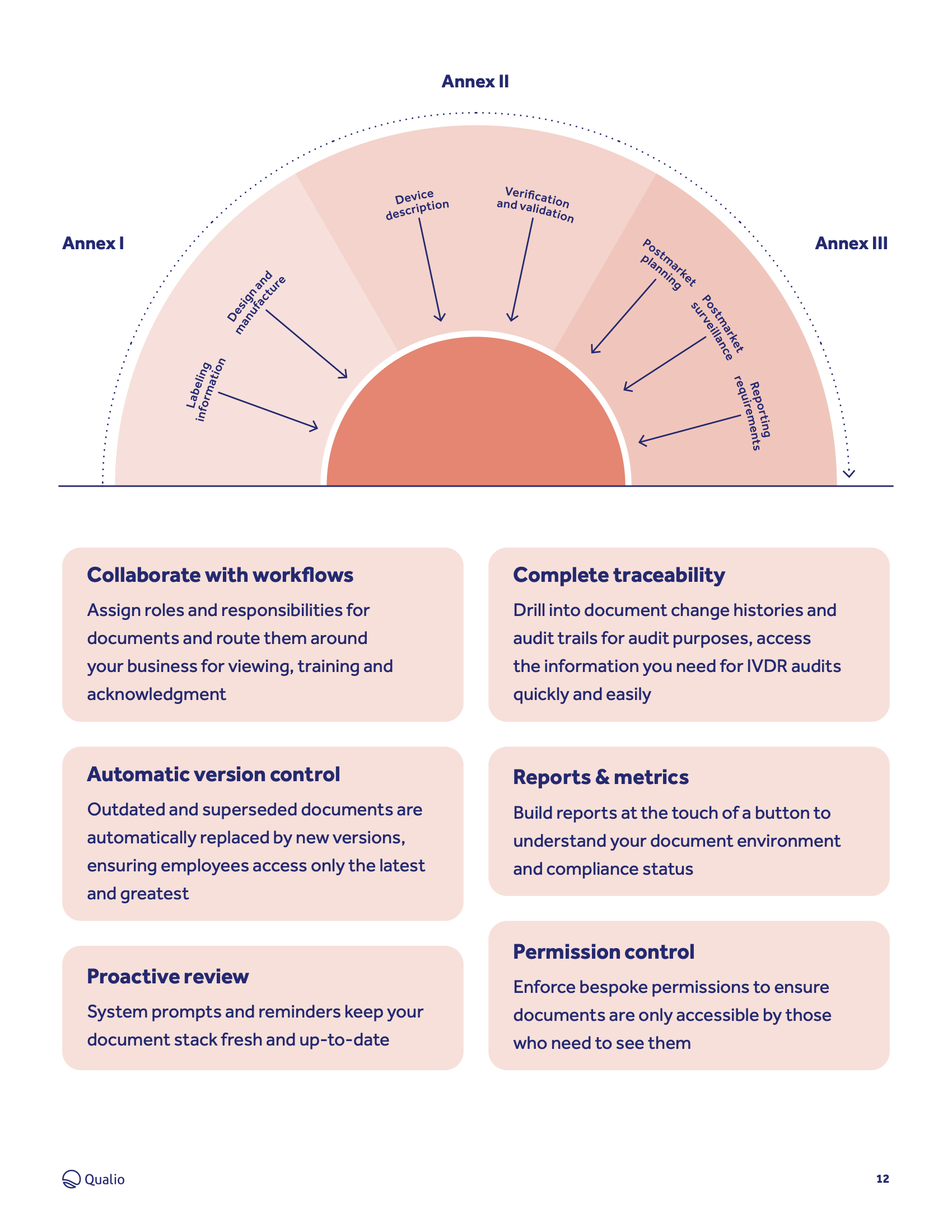
https www qualio com hubfs EU IVDR Annex requirements png - EU IVDR Software Datasheet EU IVDR Annex Requirements