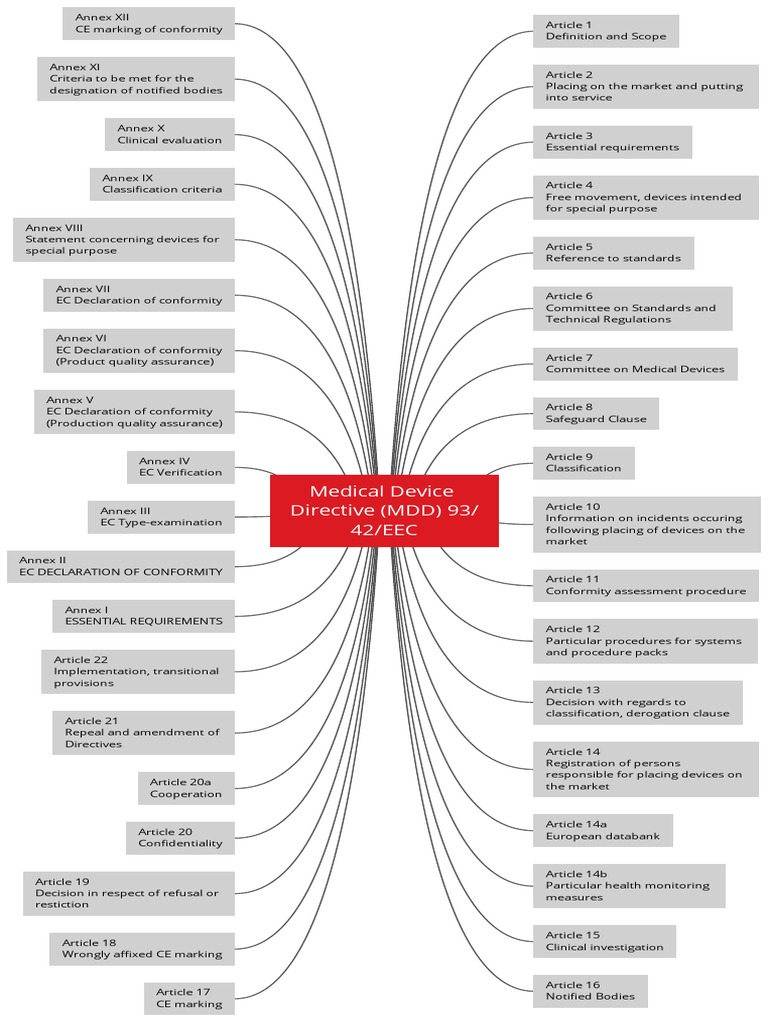
Last update images today Medical Device Directive Mdd 9342eec





























https qwivy com storage covers 2208 png - NUR 2392 NUR2392 Multidimensional Care II MDC 2 Exam 2 Blueprint 2208 https qwivy com storage covers 2091 png - NUR 2392 NUR2392 Test 1 Review Latest 2021 2022 Multidimensional 2091
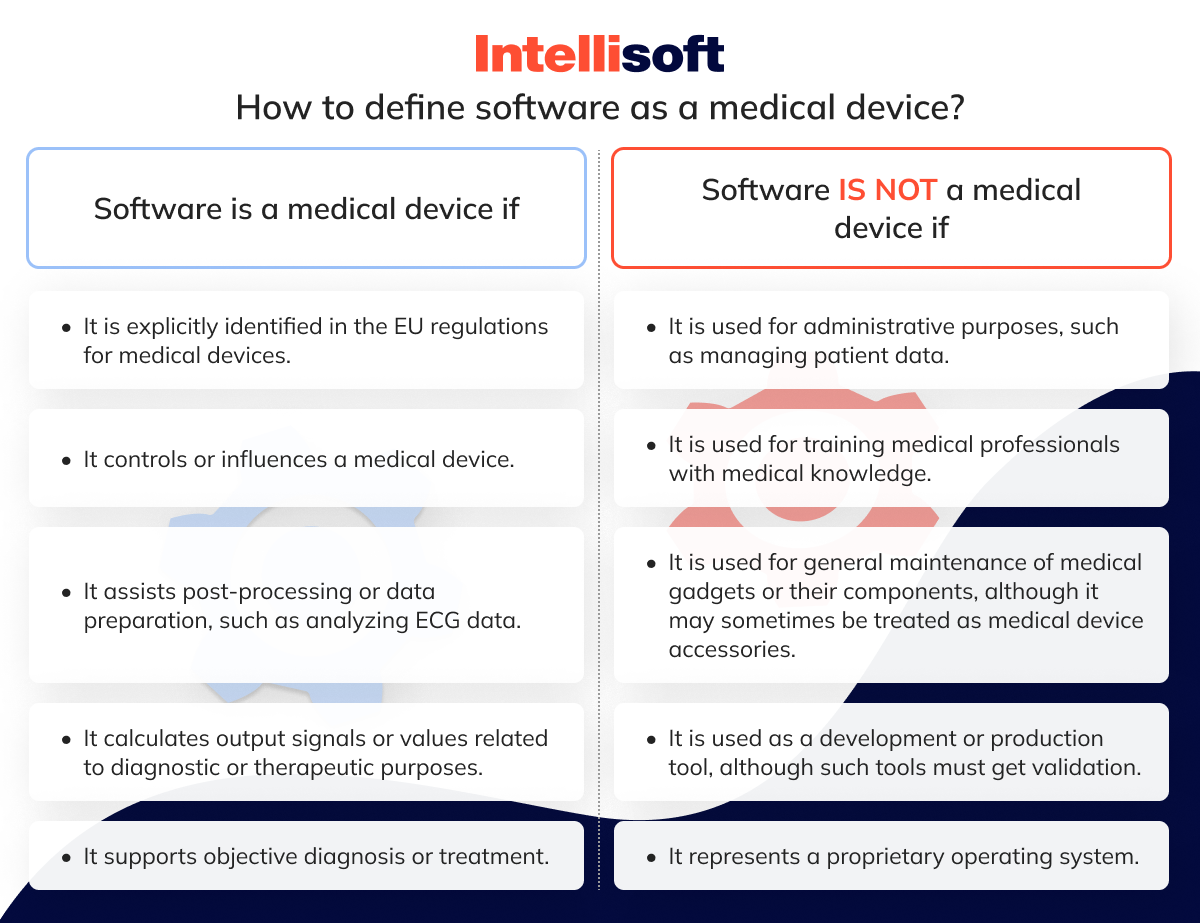
https qwivy com storage covers 2209 png - NUR 2392 NUR2392 Multidimensional Care II MDC 2 Exam 1 Blueprint 2209 https medicaldevicehq com wp content uploads 2022 09 MDR Medical Device Regulation article1 png - MDR Article 22 Medical Device HQ MDR Medical Device Regulation Article1 https intellisoft io wp content uploads 2024 03 3 33 png - EU Medical Device Regulation Compliance And Updates In 2024 3 33
http www researchandmarkets com product images 8401 8401985 500px jpg webinar jpg - EU Medical Device Regulation MDR Updated CE Marking Process And ISO Webinar https www proxomed com fileadmin processed a 3 csm EG Zertifikat Kl IIa proxomed engl 2024 05 26 2193d1bbce png - mdr proxomed Medical Device Guidelines The New MDR From May 2021 Csm EG Zertifikat Kl IIa Proxomed Engl 2024 05 26 2193d1bbce
https images squarespace cdn com content v1 5e92b50233b9b4020c550428 1587180281833 PKZC7LD4PB8BDTN6D05E MDD vs MDR png - MDR Guidance Medical Device Regulatory Guide MDD Vs MDR
https imgv2 1 f scribdassets com img document 674420344 original cdefae8a87 1703871448 - Ce Hironic MDD 93 42 Eec 2020 PDF 1703871448https qwivy com storage covers 2208 png - NUR 2392 NUR2392 Multidimensional Care II MDC 2 Exam 2 Blueprint 2208
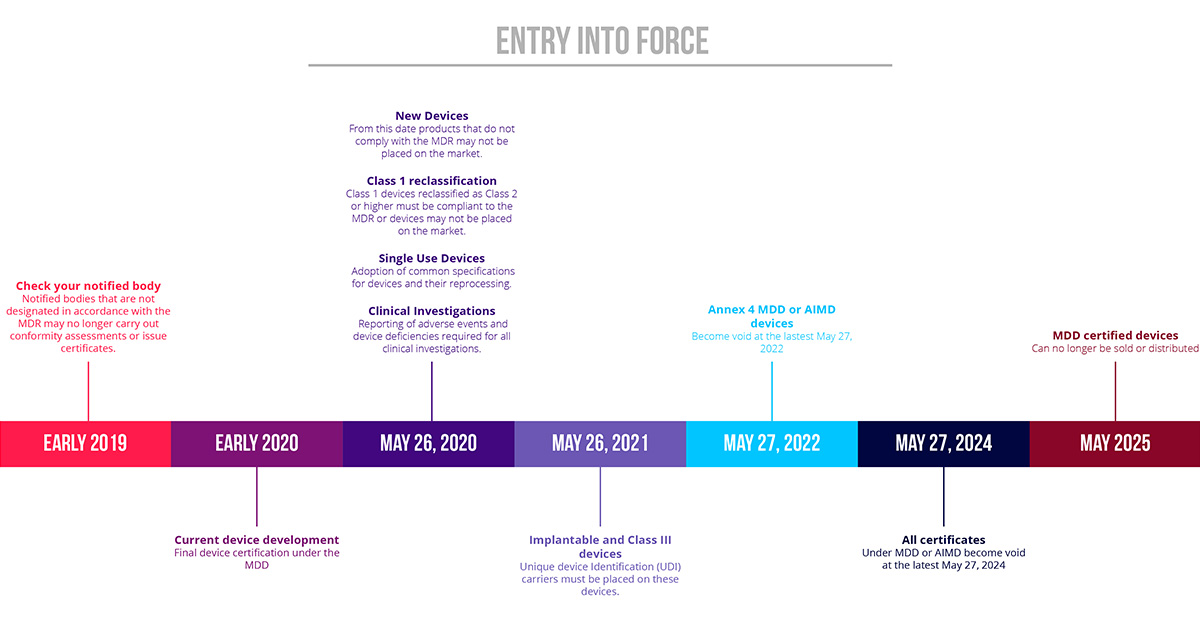
https learn marsdd com wp content uploads 2022 02 transition timeline MDD MDR 20220204 1536x631 jpg - Medical Device Regulations Classification Submissions Canada US EU Transition Timeline MDD MDR 20220204 1536x631 https www bureauveritas co kr sites g files zypfnx476 files 2019 11 chair 2584260 1920 jpg - CE MEDICAL DEVICE DIRECTIVE 93 42 EEC MDD South Korea Chair 2584260 1920
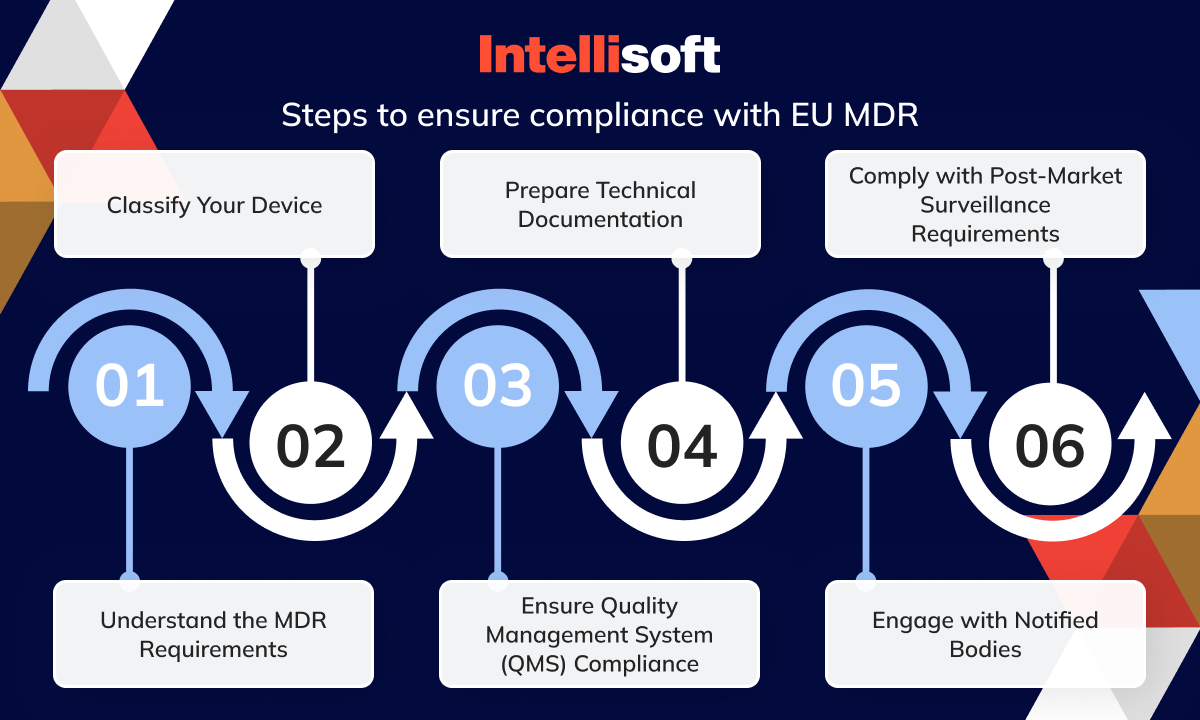
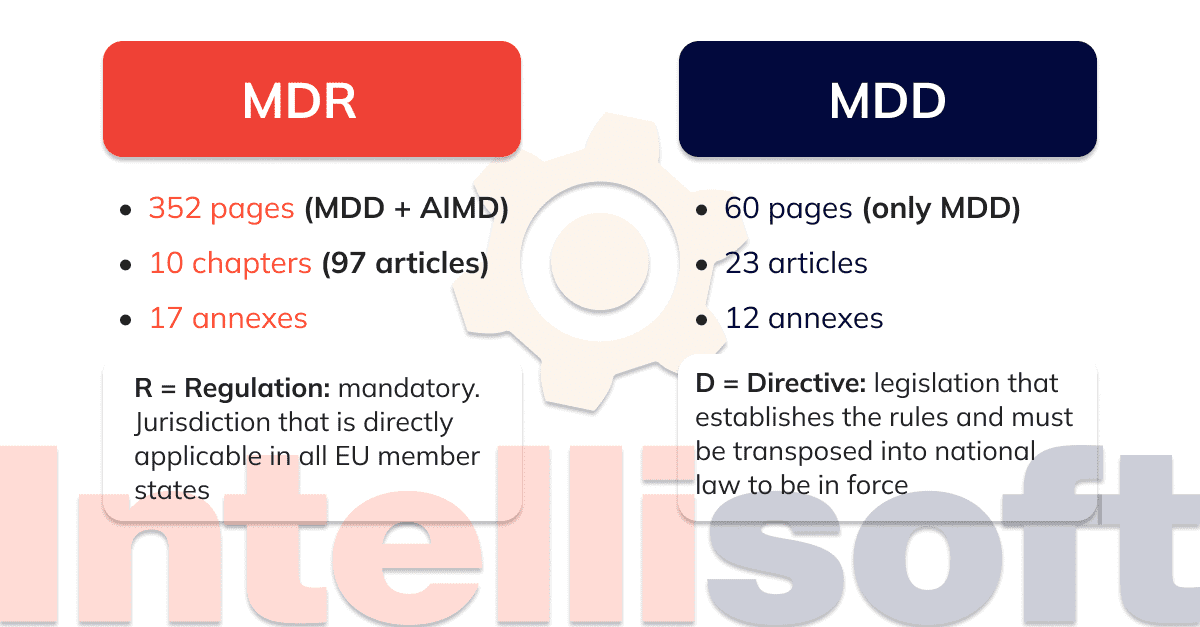
https intellisoft io wp content uploads 2024 03 4 20 png - EU Medical Device Regulation Compliance And Updates In 2024 4 20 https a storyblok com f 84976 4800x2700 e78f69dc7b medical device directive to medical device regulation og png - Medical Device Translation What Do You Need To Know Medical Device Directive To Medical Device Regulation Og https qwivy com storage covers 2209 png - NUR 2392 NUR2392 Multidimensional Care II MDC 2 Exam 1 Blueprint 2209
https cemarking net wp content uploads 2022 05 electronic manuals medical devices jpg - EU 2021 2226 Explained Electronic Manuals For Medical Devices Electronic Manuals Medical Devices https d2dzik4ii1e1u6 cloudfront net images lexology static 45a5ae32 4d5f 4a2b bfdd ecec2c265e38 PNG - Medical Devices Implementation Of Future Regulations Lexology 45a5ae32 4d5f 4a2b Bfdd Ecec2c265e38.PNG
https qwivy com storage covers 2091 png - NUR 2392 NUR2392 Test 1 Review Latest 2021 2022 Multidimensional 2091
https static wixstatic com media 01d489 019e31a0287d46c491f2b85cab7a3c5a mv2 png v1 fit w 960 h 640 al c q 80 file png - mhra mdr ivdr regulations period guidance directives transition The Interactive Guide Under The New EU Regulations On Medical Devices File https intellisoft io wp content uploads 2024 03 3 33 png - EU Medical Device Regulation Compliance And Updates In 2024 3 33
https a storyblok com f 84976 4800x2700 e78f69dc7b medical device directive to medical device regulation og png - Medical Device Translation What Do You Need To Know Medical Device Directive To Medical Device Regulation Og https qwivy com storage covers 2091 png - NUR 2392 NUR2392 Test 1 Review Latest 2021 2022 Multidimensional 2091
https qwivy com storage covers 2208 png - NUR 2392 NUR2392 Multidimensional Care II MDC 2 Exam 2 Blueprint 2208 https imgv2 1 f scribdassets com img document 674420344 original cdefae8a87 1703871448 - Ce Hironic MDD 93 42 Eec 2020 PDF 1703871448https www tuvsud com media global images resource centre infographics resource centre tuv sud mdr infographic jpg - Infographic The New Medical Device Regulation T V S D In India Tuv Sud Mdr Infographic
https www cambridge design com wp content uploads 2021 07 LI jpg - Medical Devices Regulation Are You Ready For 2020 CDP LI https qwivy com storage covers 2209 png - NUR 2392 NUR2392 Multidimensional Care II MDC 2 Exam 1 Blueprint 2209
https intellisoft io wp content uploads 2024 03 4 20 png - EU Medical Device Regulation Compliance And Updates In 2024 4 20