Last update images today Medical Device Regulatory Pathway
















https kvalito ch wp content uploads 2020 04 7 1 png - regulatory legislation pathways classification structured Medical Devices US And Chinese Legislation Kvalito 7 1 https www mystrategist com binary file transfer 040 002 runozc png - Market Pathways Medical Device Reform In Europe Where Do We Go From Runozc
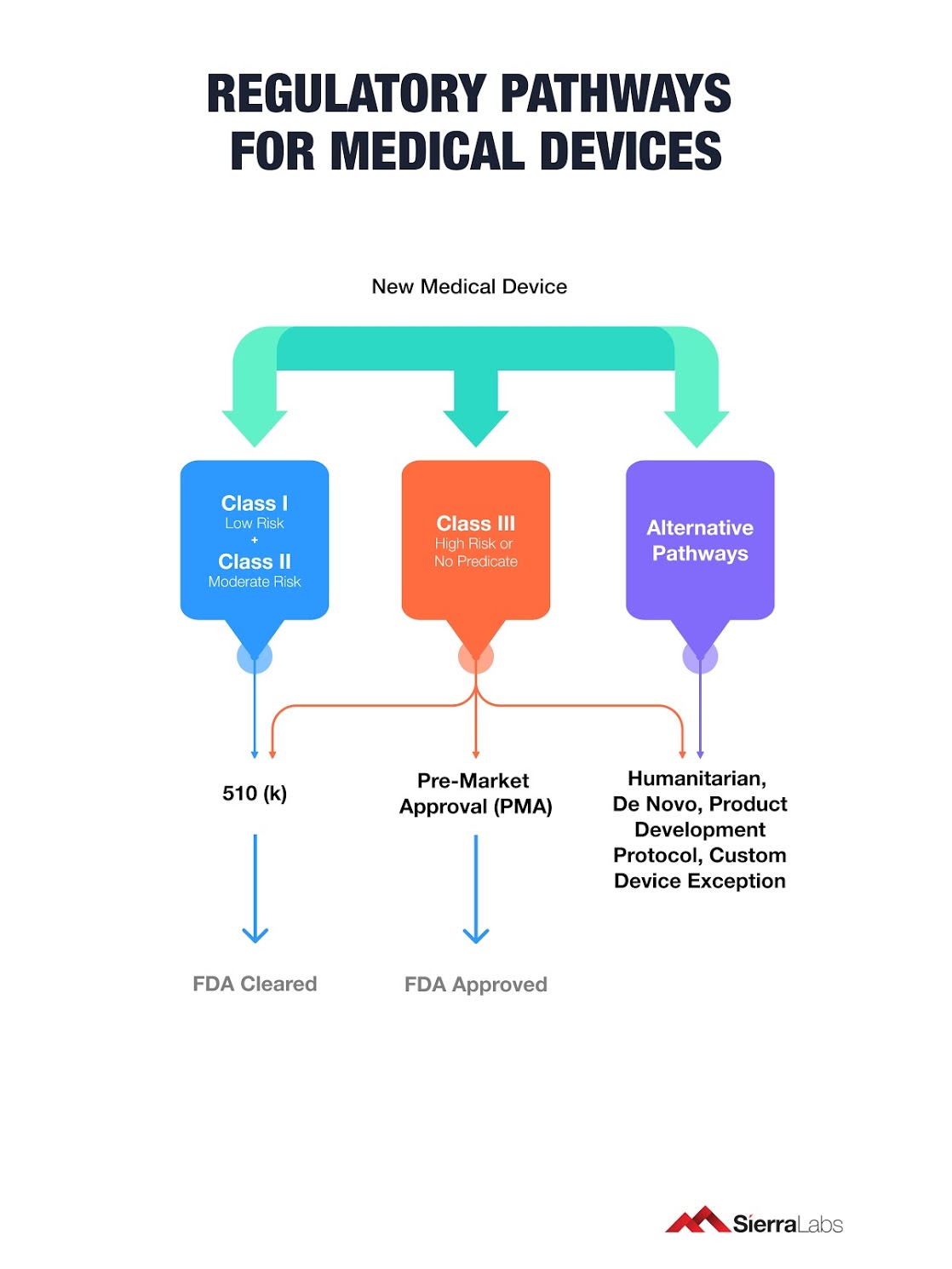
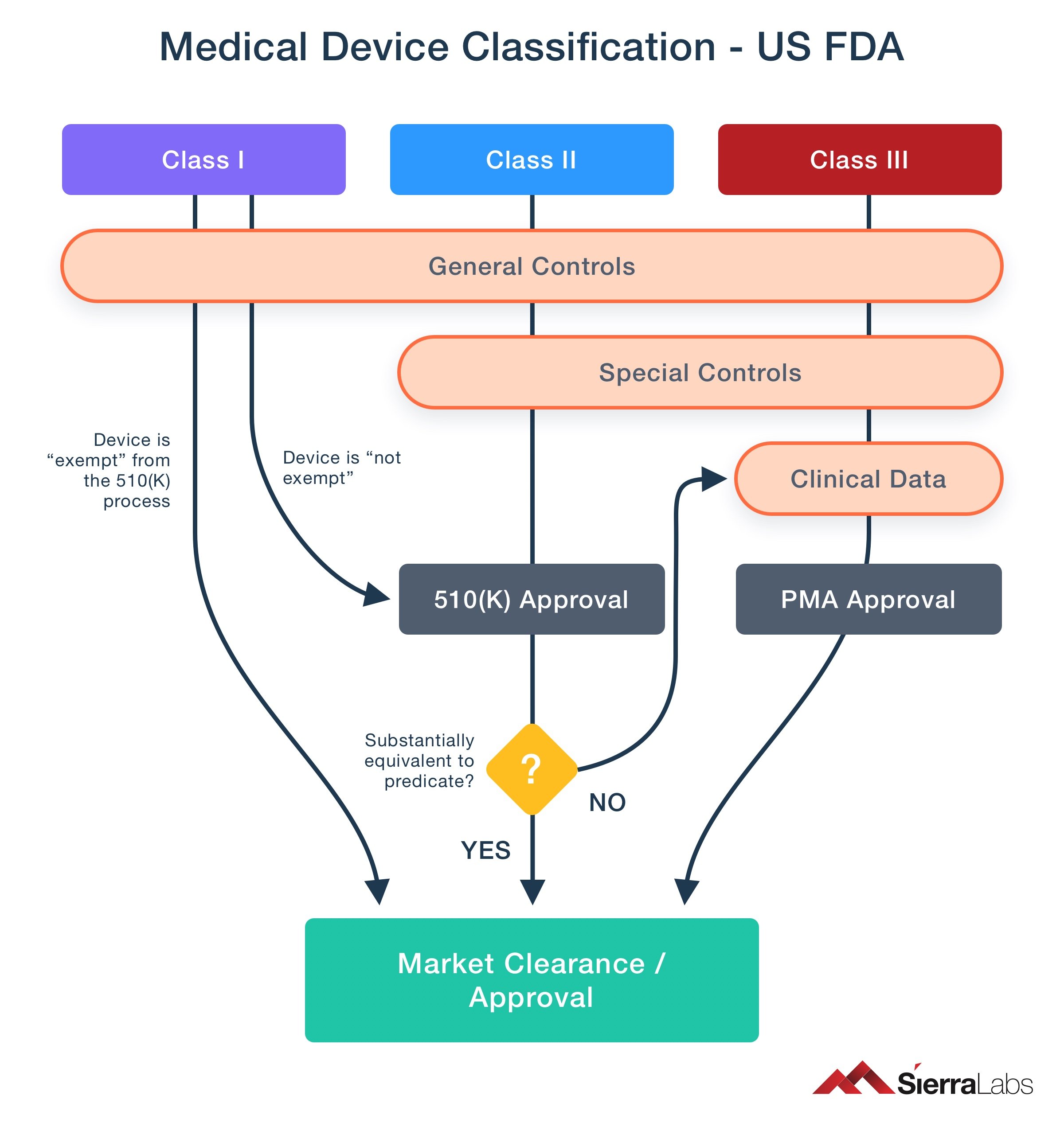
https learn marsdd com wp content uploads 2022 02 transition timeline MDD MDR 20220204 1024x421 jpg - Medical Device Regulations Classification Submissions Canada US EU Transition Timeline MDD MDR 20220204 1024x421 https medregs blog gov uk wp content uploads sites 195 2024 02 MedTech roadmap 2 png - Med Tech Regulatory Reform The First Steps Towards A New Framework For MedTech Roadmap 2 https blog sierralabs com hs fs hubfs 4 1 jpg - regulatory pma classification approval premarket ISO 13485 Regulatory Requirements On Medical Devices 4 1
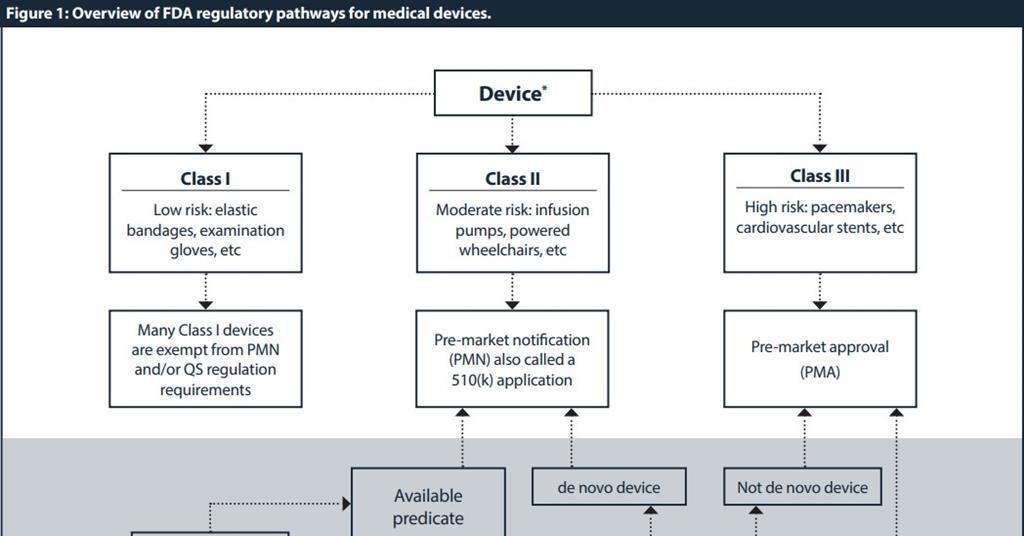
https startoonlabs com blogs static images blog eleven jpg - Startoon Labs Blog Eleven https ds982gbwnvyso cloudfront net Pictures 1024x536 2 5 0 250 may2019fig1 312671 jpg - FDA Regulatory Pathways For Medical Devices Journal Regulatory 250 May2019fig1 312671
https www researchgate net publication 369348910 figure fig5 AS 11431281127783809 1679149193486 Medical device approval pathway in India 27 ppm - Medical Device Approval Pathway In India 27 Download Scientific Medical Device Approval Pathway In India 27.ppm
https www researchgate net publication 369348910 figure fig5 AS 11431281127783809 1679149193486 Medical device approval pathway in India 27 ppm - Medical Device Approval Pathway In India 27 Download Scientific Medical Device Approval Pathway In India 27.ppmhttps www mastertrial com wp content uploads 2022 11 TR EU 141 MDR Roadmap Rev 4 0 completed script 20200702 final 1 1 800x450 jpg - Introduction To Medical Device Regulation Roadmap Orientation TR EU 141 MDR Roadmap Rev.4.0 Completed Script 20200702 Final 1 1 800x450
https www fdanews com ext resources Book Covers NEW Medical Device Pathways 500 jpg - Medical Device Pathways Navigating FDA Roads To Market FDAnews Medical Device Pathways 500 https www pathologyinpractice com api file 61031 - MHRA Publishes Regulatory Roadmap For Medical Devices 61031
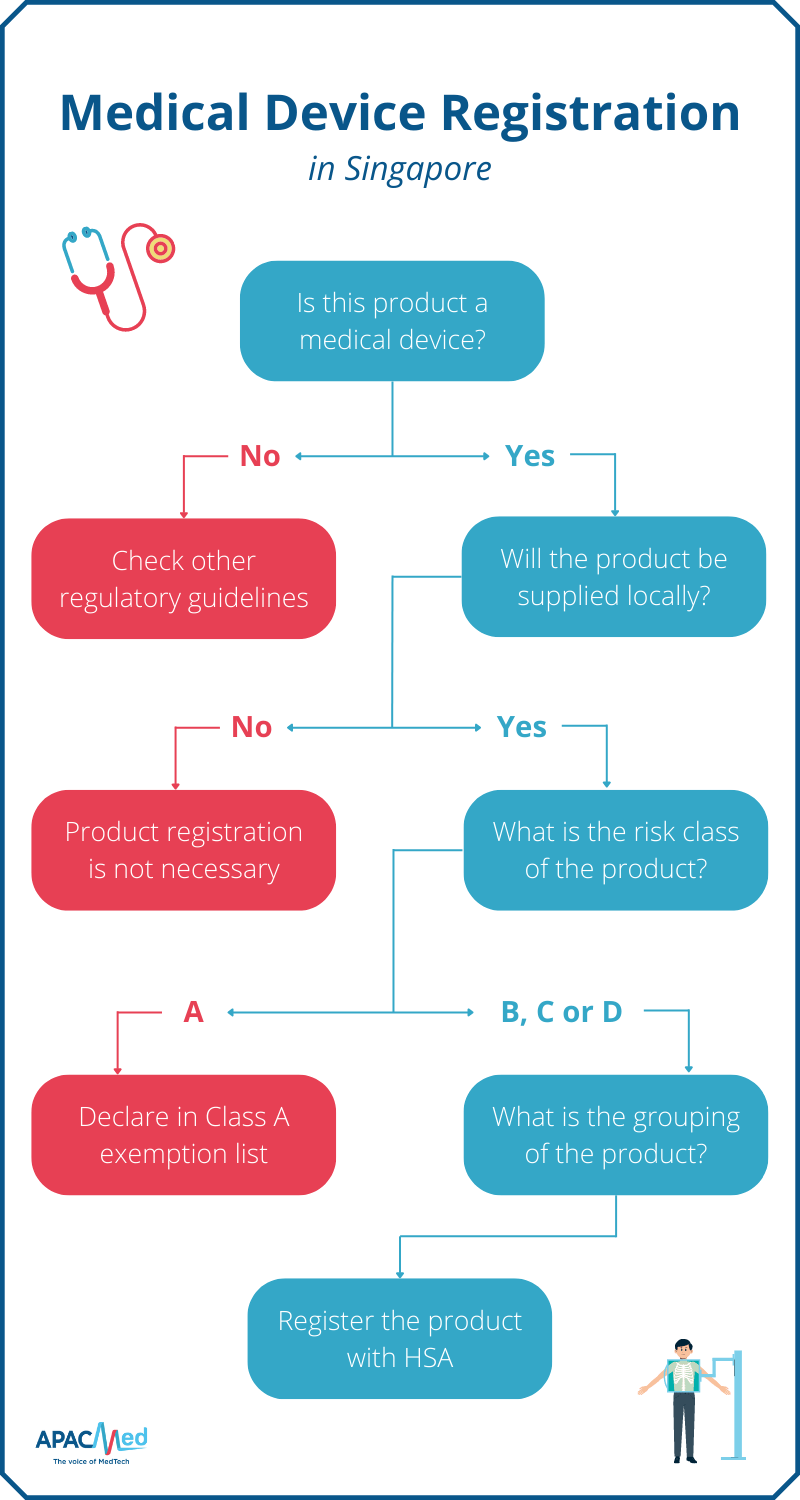
https medicaldeviceslegal files wordpress com 2022 06 new comitology rules art 291 tfeu png - Managing The 2024 MDR Danger Zone And Outlines Of A Potential New Comitology Rules Art. 291 Tfeu https images ctfassets net o78em1y1w4i4 739pwYjARKZJxrLYvd5rPV 9dc7005f52dc541971c3fefd46e6dd58 GettyImages 501868861 jpg - Medical Device Regulation Landscape And Trends GettyImages 501868861 https apacmed org content uploads 2022 10 Registration Pathways for Medical Device Regulation in Singapore png - Medical Device Regulation Importance And Examples In APAC 2022 Registration Pathways For Medical Device Regulation In Singapore
https www opticianonline net media hccjfvzm ann20blackmore20osa20regulatory20consultant final20edit jpg - Device Regulation Extended To 2024 Ann20blackmore20osa20regulatory20consultant Final20edit https www researchgate net publication 369348910 figure fig4 AS 11431281127774549 1679149192936 Medical device approval pathway in Europe 23 ppm - Medical Device Approval Pathway In Europe 23 Download Scientific Medical Device Approval Pathway In Europe 23.ppm
https kvalito ch wp content uploads 2020 04 7 1 png - regulatory legislation pathways classification structured Medical Devices US And Chinese Legislation Kvalito 7 1
https images ctfassets net o78em1y1w4i4 739pwYjARKZJxrLYvd5rPV 9dc7005f52dc541971c3fefd46e6dd58 GettyImages 501868861 jpg - Medical Device Regulation Landscape And Trends GettyImages 501868861 https casusconsulting com wp content uploads 2024 01 UK MHRA 2024 2025 Medical Device Roadmap png - UK MHRA 2024 2025 Medical Device Regulation Plan Casus Consulting UK MHRA 2024 2025 Medical Device Roadmap
https www mystrategist com binary file transfer 040 002 runozc png - Market Pathways Medical Device Reform In Europe Where Do We Go From Runozc https andamanmed com wp content uploads 2023 11 Shutterstock 2352695679 scaled jpg - Malaysia Standards For Medical Device Registration 2024 Shutterstock 2352695679 Scaled
https kvalito ch wp content uploads 2020 04 7 1 png - regulatory legislation pathways classification structured Medical Devices US And Chinese Legislation Kvalito 7 1 https casusconsulting com wp content uploads 2024 01 UK MHRA 2024 2025 Medical Device Roadmap png - UK MHRA 2024 2025 Medical Device Regulation Plan Casus Consulting UK MHRA 2024 2025 Medical Device Roadmap https medicaldeviceslegal files wordpress com 2022 06 schermafbeelding 2022 06 19 om 23 51 20 png - Axon Lawyers Managing The 2024 MDR Danger Zone And Outlines Of A Schermafbeelding 2022 06 19 Om 23.51.20
https images ctfassets net o78em1y1w4i4 739pwYjARKZJxrLYvd5rPV 9dc7005f52dc541971c3fefd46e6dd58 GettyImages 501868861 jpg - Medical Device Regulation Landscape And Trends GettyImages 501868861 https www medicaldevice network com wp content uploads sites 23 2023 12 shutterstock 2344018595 1 jpg - Regulatory Changes In The US And UK To Watch In 2024 Medical Device Shutterstock 2344018595 1
http acmebiotechstc weebly com uploads 4 8 8 0 48804951 3735275 orig png - 3735275 Orig